T cells regulate lymph node-resident ILC populations in a tissue and subset-specific way
- PMID: 33665576
- PMCID: PMC7907429
- DOI: 10.1016/j.isci.2021.102158
T cells regulate lymph node-resident ILC populations in a tissue and subset-specific way
Abstract
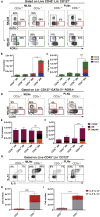
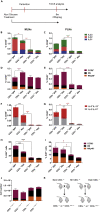
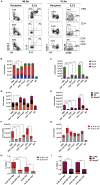
Innate lymphoid cells (ILCs) have been shown to be significantly affected in the small intestine lamina propria and secondary lymphoid organs (SLOs) of conventional lymphopenic mice. How ILCs are regulated by adaptive immunity in SLOs remains unclear. In T cell-deficient mice, ILC2s are significantly increased in the mesenteric lymph nodes (MLNs) at the expense of CCR6+ ILC3s, which are nonetheless increased in the peripheral lymph nodes (PLNs). Here, we show that T cells regulate lymph node-resident ILCs in a tissue- and subset-specific way. First, reducing microbial colonization from birth restored CCR6+ ILC3s in the MLNs of T cell-deficient mice. In contrast, T cell reconstitution resulted in the contraction of both MLNs ILC2s and PLNs ILC3s, whereas antagonizing microbial colonization from birth had no impact on these populations. Finally, the accumulation of MLNs ILC2s was partly regulated by T cells through stroma-derived IL-33.
Keywords: Components of the Immune System; Immunology; Microbiome.
© 2021 The Authors.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
Similar articles
-
Innate Lymphoid Cells in the Maternal and Fetal Compartments.Front Immunol. 2018 Oct 26;9:2396. doi: 10.3389/fimmu.2018.02396. eCollection 2018. Front Immunol. 2018. PMID: 30416502 Free PMC article. Review.
-
Peripheral lymph nodes contain migratory and resident innate lymphoid cell populations.Sci Immunol. 2019 May 31;4(35):eaau8082. doi: 10.1126/sciimmunol.aau8082. Sci Immunol. 2019. PMID: 31152090 Free PMC article.
-
Zbtb1 controls NKp46+ ROR-gamma-T+ innate lymphoid cell (ILC3) development.Oncotarget. 2017 Jul 27;8(34):55877-55888. doi: 10.18632/oncotarget.19645. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915559 Free PMC article.
-
Tissue-Specific Induction of CCR6 and Nrp1 During Early CD4+ T Cell Differentiation.Eur J Microbiol Immunol (Bp). 2016 Aug 23;6(3):219-226. doi: 10.1556/1886.2016.00018. eCollection 2016 Sep 29. Eur J Microbiol Immunol (Bp). 2016. PMID: 27766171 Free PMC article.
-
Innate lymphoid cells in secondary lymphoid organs.Immunol Rev. 2016 May;271(1):185-99. doi: 10.1111/imr.12407. Immunol Rev. 2016. PMID: 27088915 Review.
Cited by
-
Group 2 innate lymphocytes protect the balance between autophagy and apoptosis in cardiomyocytes during sepsis-induced cardiac injury.Sci Rep. 2024 Oct 23;14(1):25011. doi: 10.1038/s41598-024-76606-4. Sci Rep. 2024. PMID: 39443633 Free PMC article.
-
The Role of Innate Lymphoid Cells in Cancer Development and Immunotherapy.Front Cell Dev Biol. 2022 Apr 26;10:803563. doi: 10.3389/fcell.2022.803563. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35557940 Free PMC article. Review.
-
The emerging role of group 3 innate lymphoid cells in the neonate: interaction with the maternal and neonatal microbiome.Oxf Open Immunol. 2021 May 12;2(1):iqab009. doi: 10.1093/oxfimm/iqab009. eCollection 2021. Oxf Open Immunol. 2021. PMID: 34151271 Free PMC article. Review.
References
-
- Bordon Y. Mucosal immunology: ILCs broker peace deals in the gut. Nat. Rev. Immunol. 2013;13:473. - PubMed
-
- Cherrier M., Ramachandran G., Golub R. The interplay between innate lymphoid cells and T cells. Mucosal Immunol. 2020;13:732–742. - PubMed
-
- Cupedo T., Kraal G., Mebius R.E. The role of CD45+CD4+CD3- cells in lymphoid organ development. Immunol. Rev. 2002;189:41–50. - PubMed
-
- Ebbo M., Crinier A., Vély F., Vivier E. Innate lymphoid cells: major players in inflammatory diseases. Nat. Rev. Immunol. 2017;17:665–678. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

