Pharmacologic Targeting of BET Proteins Attenuates Hyperuricemic Nephropathy in Rats
- PMID: 33664670
- PMCID: PMC7921804
- DOI: 10.3389/fphar.2021.636154
Pharmacologic Targeting of BET Proteins Attenuates Hyperuricemic Nephropathy in Rats
Abstract
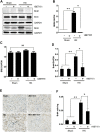
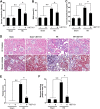
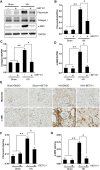
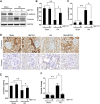
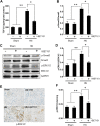
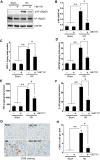
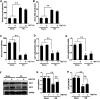
Hyperuricemia is an independent risk factor for renal damage and promotes the progression of chronic kidney disease. In this study, we investigated the effect of I-BET151, a small-molecule inhibitor targeting the bromodomain and extraterminal (BET) proteins, on the development of hyperuricemic nephropathy (HN), and the mechanisms involved. Expression levels of bromodomain-containing protein 2 and 4, but not 3 were increased in the kidney of rats with HN; administration of I-BET151 effectively prevented renal dysfunction, decreased urine microalbumin, and attenuated renal fibrosis as indicated by reduced activation of renal interstitial fibroblasts and expression of fibronectin and collagen I in HN rats. Mechanistic studies show that I-BET151 treatment inhibited transition of renal epithelial cells to a mesenchymal cell type as evidenced by preservation of E-cadherin and reduction of vimentin expression. This was coincident with reduced expression of TGF-β1 and dephosphorylation of Smad3 and ERK1/2. I-BET151 was also effective in inhibiting phosphorylation of NF-κB, expression of multiple cytokines and chemokines, and infiltration of macrophages to the injured kidney. Although there were increased serum levels of uric acid and xanthine oxidase, an enzyme that catalyzes production of uric acid, and decreased expression of renal organic anion transporter 1 and 3 that promote urate excretion in the model of HN, and reduced expression levels of urine uric acid, I-BET151 treatment did not affect these responses. Collectively, our results indicate that I-BET151 alleviates HN by inhibiting epithelial to mesenchymal transition and inflammation in association with blockade of TGF-β, ERK1/2 and NF-κB signaling.
Keywords: I-BET151; bromodomain and extra-terminal proteins; epithelial-to-mesenchymal transition; hyperuricemic nephropathy; inflammation; renal fibrosis.
Copyright © 2021 Xiong, Deng, Wang, Shao, Zhou, Zou and Zhuang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Pharmacologic targeting ERK1/2 attenuates the development and progression of hyperuricemic nephropathy in rats.Oncotarget. 2017 May 16;8(20):33807-33826. doi: 10.18632/oncotarget.16995. Oncotarget. 2017. PMID: 28442634 Free PMC article.
-
EGF Receptor Inhibition Alleviates Hyperuricemic Nephropathy.J Am Soc Nephrol. 2015 Nov;26(11):2716-29. doi: 10.1681/ASN.2014080793. Epub 2015 Mar 18. J Am Soc Nephrol. 2015. PMID: 25788532 Free PMC article.
-
Pharmacological inhibition of Src family kinases attenuates hyperuricemic nephropathy.Front Pharmacol. 2024 Mar 21;15:1352730. doi: 10.3389/fphar.2024.1352730. eCollection 2024. Front Pharmacol. 2024. PMID: 38576481 Free PMC article.
-
Natural flavonol fisetin attenuated hyperuricemic nephropathy via inhibiting IL-6/JAK2/STAT3 and TGF-β/SMAD3 signaling.Phytomedicine. 2021 Jul;87:153552. doi: 10.1016/j.phymed.2021.153552. Epub 2021 Mar 22. Phytomedicine. 2021. PMID: 33994251
-
Anticancer Effects of I-BET151, an Inhibitor of Bromodomain and Extra-Terminal Domain Proteins.Front Oncol. 2021 Sep 2;11:716830. doi: 10.3389/fonc.2021.716830. eCollection 2021. Front Oncol. 2021. PMID: 34540687 Free PMC article. Review.
Cited by
-
Histone Acetylation and Modifiers in Renal Fibrosis.Front Pharmacol. 2022 Apr 26;13:760308. doi: 10.3389/fphar.2022.760308. eCollection 2022. Front Pharmacol. 2022. PMID: 35559244 Free PMC article. Review.
-
Combination of allopurinol with Dahuang Mudan Tang significantly improve kidney function and alleviate oxidative stress and inflammation of chronic kidney disease stage Ⅰ-Ⅲ patients with hyperuricemia.J Tradit Chin Med. 2024 Feb;44(1):182-187. doi: 10.19852/j.cnki.jtcm.20231121.001. J Tradit Chin Med. 2024. PMID: 38213253 Free PMC article.
-
The Rho kinase signaling pathway participates in tubular mitochondrial oxidative injury and apoptosis in uric acid nephropathy.J Int Med Res. 2021 Jun;49(6):3000605211021752. doi: 10.1177/03000605211021752. J Int Med Res. 2021. PMID: 34167354 Free PMC article.
-
BRD4: an effective target for organ fibrosis.Biomark Res. 2024 Aug 30;12(1):92. doi: 10.1186/s40364-024-00641-6. Biomark Res. 2024. PMID: 39215370 Free PMC article. Review.
-
Type IV Collagen and SOX9 Are Molecular Targets of BET Inhibition in Experimental Glomerulosclerosis.Int J Mol Sci. 2022 Dec 28;24(1):486. doi: 10.3390/ijms24010486. Int J Mol Sci. 2022. PMID: 36613933 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

