Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models
- PMID: 33663578
- PMCID: PMC7934466
- DOI: 10.1186/s13024-021-00434-7
Enhancing GABAergic signaling ameliorates aberrant gamma oscillations of olfactory bulb in AD mouse models
Abstract
Background: Before the deposition of amyloid-beta plaques and the onset of learning memory deficits, patients with Alzheimer's disease (AD) experience olfactory dysfunction, typified by a reduced ability to detect, discriminate, and identify odors. Rodent models of AD, such as the Tg2576 and APP/PS1 mice, also display impaired olfaction, accompanied by aberrant in vivo or in vitro gamma rhythms in the olfactory pathway. However, the mechanistic relationships between the electrophysiological, biochemical and behavioral phenomena remain unclear.
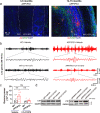
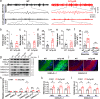
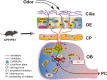
Methods: To address the above issues in AD models, we conducted in vivo measurement of local field potential (LFP) with a combination of in vitro electro-olfactogram (EOG), whole-cell patch and field recordings to evaluate oscillatory and synaptic function and pharmacological regulation in the olfactory pathway, particularly in the olfactory bulb (OB). Levels of protein involved in excitation and inhibition of the OB were investigated by western blotting and fluorescence staining, while behavioral studies assessed olfaction and memory function.
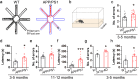
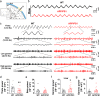
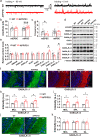
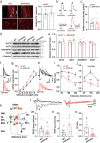
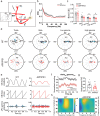
Results: LFP measurements demonstrated an increase in gamma oscillations in the OB accompanied by altered olfactory behavior in both APP/PS1 and 3xTg mice at 3-5 months old, i.e. an age before the onset of plaque formation. Fewer olfactory sensory neurons (OSNs) and a reduced EOG contributed to a decrease in the excitatory responses of M/T cells, suggesting a decreased ability of M/T cells to trigger interneuron GABA release indicated by altered paired-pulse ratio (PPR), a presynaptic parameter. Postsynaptically, there was a compensatory increase in levels of GABAAR α1 and β3 subunits and subsequent higher amplitude of inhibitory responses. Strikingly, the GABA uptake inhibitor tiagabine (TGB) ameliorated abnormal gamma oscillations and levels of GABAAR subunits, suggesting a potential therapeutic strategy for early AD symptoms. These findings reveal increased gamma oscillations in the OB as a core indicator prior to onset of AD and uncover mechanisms underlying aberrant gamma activity in the OB.
Conclusions: This study suggests that the concomitant dysfunction of both olfactory behavior and gamma oscillations have important implications for early AD diagnosis: in particular, awareness of aberrant GABAergic signaling mechanisms might both aid diagnosis and suggest therapeutic strategies for olfactory damage in AD.
Keywords: 3xTg; APP/PS1; Alzheimer’s disease; GABAAR; Gamma oscillations; Olfactory bulb; Tiagabine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
ErbB4 deficiency exacerbates olfactory dysfunction in an early-stage Alzheimer's disease mouse model.Acta Pharmacol Sin. 2024 Dec;45(12):2497-2512. doi: 10.1038/s41401-024-01332-6. Epub 2024 Jul 9. Acta Pharmacol Sin. 2024. PMID: 38982150
-
An early dysregulation of FAK and MEK/ERK signaling pathways precedes the β-amyloid deposition in the olfactory bulb of APP/PS1 mouse model of Alzheimer's disease.J Proteomics. 2016 Oct 4;148:149-58. doi: 10.1016/j.jprot.2016.07.032. Epub 2016 Aug 3. J Proteomics. 2016. PMID: 27498392
-
GABAA receptor agonist muscimol rescues inhibitory microcircuit defects in the olfactory bulb and improves olfactory function in APP/PS1 transgenic mice.Neurobiol Aging. 2021 Dec;108:47-57. doi: 10.1016/j.neurobiolaging.2021.08.003. Epub 2021 Aug 9. Neurobiol Aging. 2021. PMID: 34507271
-
A potential biomarker of preclinical Alzheimer's disease: The olfactory dysfunction and its pathogenesis-based neural circuitry impairments.Neurosci Biobehav Rev. 2022 Jan;132:857-869. doi: 10.1016/j.neubiorev.2021.11.009. Epub 2021 Nov 20. Neurosci Biobehav Rev. 2022. PMID: 34810025 Review.
-
Unsupervised excitation: GABAergic dysfunctions in Alzheimer's disease.Brain Res. 2019 Mar 15;1707:216-226. doi: 10.1016/j.brainres.2018.11.042. Epub 2018 Nov 29. Brain Res. 2019. PMID: 30503351 Review.
Cited by
-
Amelioration of olfactory dysfunction in a mouse model of Parkinson's disease via enhancing GABAergic signaling.Cell Biosci. 2023 Jun 3;13(1):101. doi: 10.1186/s13578-023-01049-9. Cell Biosci. 2023. PMID: 37270503 Free PMC article.
-
Precocious emergence of cognitive and synaptic dysfunction in 3xTg-AD mice exposed prenatally to ethanol.Alcohol. 2023 Mar;107:56-72. doi: 10.1016/j.alcohol.2022.08.003. Epub 2022 Aug 28. Alcohol. 2023. PMID: 36038084 Free PMC article.
-
Biocytin-Labeling in Whole-Cell Recording: Electrophysiological and Morphological Properties of Pyramidal Neurons in CYLD-Deficient Mice.Molecules. 2023 May 15;28(10):4092. doi: 10.3390/molecules28104092. Molecules. 2023. PMID: 37241833 Free PMC article.
-
Deficiency of the CYLD Impairs Fear Memory of Mice and Disrupts Neuronal Activity and Synaptic Transmission in the Basolateral Amygdala.Front Cell Neurosci. 2021 Sep 17;15:740165. doi: 10.3389/fncel.2021.740165. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34602983 Free PMC article.
-
Resveratrol Attenuates Chronic Unpredictable Mild Stress-Induced Alterations in the SIRT1/PGC1α/SIRT3 Pathway and Associated Mitochondrial Dysfunction in Mice.Mol Neurobiol. 2023 Sep;60(9):5102-5116. doi: 10.1007/s12035-023-03395-8. Epub 2023 May 31. Mol Neurobiol. 2023. PMID: 37256428
References
-
- Goedert M, Spillantini MG. A century of Alzheimer’s disease. Science. 2006;314:777–781. - PubMed
-
- Canter RG, Penney J, Tsai LH. The road to restoring neural circuits for the treatment of Alzheimer's disease. Nature. 2016;539:187–196. - PubMed
-
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer's disease. Nature. 2016;537:50–56. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

