Spots, stripes, and spiral waves in models for static and motile cells : GTPase patterns in cells
- PMID: 33660145
- PMCID: PMC7929972
- DOI: 10.1007/s00285-021-01550-0
Spots, stripes, and spiral waves in models for static and motile cells : GTPase patterns in cells
Abstract
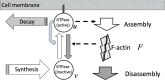
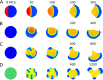
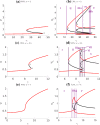
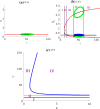
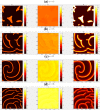
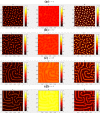
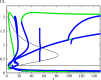
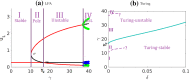
The polarization and motility of eukaryotic cells depends on assembly and contraction of the actin cytoskeleton and its regulation by proteins called GTPases. The activity of GTPases causes assembly of filamentous actin (by GTPases Cdc42, Rac), resulting in protrusion of the cell edge. Mathematical models for GTPase dynamics address the spontaneous formation of patterns and nonuniform spatial distributions of such proteins in the cell. Here we revisit the wave-pinning model for GTPase-induced cell polarization, together with a number of extensions proposed in the literature. These include introduction of sources and sinks of active and inactive GTPase (by the group of A. Champneys), and negative feedback from F-actin to GTPase activity. We discuss these extensions singly and in combination, in 1D, and 2D static domains. We then show how the patterns that form (spots, waves, and spirals) interact with cell boundaries to create a variety of interesting and dynamic cell shapes and motion.
Keywords: GTPase; Intracellular signaling; Local perturbation analysis; Pattern formation; Static and moving boundary computation; wave-pinning.
Figures


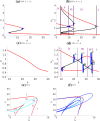

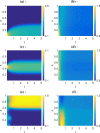
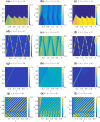
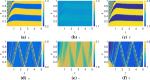
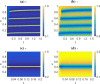
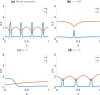
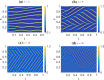
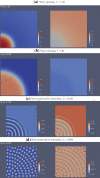




Similar articles
-
Cell Size, Mechanical Tension, and GTPase Signaling in the Single Cell.Bull Math Biol. 2020 Feb 3;82(2):28. doi: 10.1007/s11538-020-00702-5. Bull Math Biol. 2020. PMID: 32016583
-
Regimes of wave type patterning driven by refractory actin feedback: transition from static polarization to dynamic wave behaviour.Phys Biol. 2012 Aug;9(4):046005. doi: 10.1088/1478-3975/9/4/046005. Epub 2012 Jul 11. Phys Biol. 2012. PMID: 22785332 Free PMC article.
-
Visualization and molecular analysis of actin assembly in living cells.J Cell Biol. 1998 Dec 28;143(7):1919-30. doi: 10.1083/jcb.143.7.1919. J Cell Biol. 1998. PMID: 9864364 Free PMC article.
-
Ras-related GTPases and the cytoskeleton.Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
-
Actin dynamics in cell migration.Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review.
Cited by
-
From actin waves to mechanism and back: How theory aids biological understanding.Elife. 2023 Jul 10;12:e87181. doi: 10.7554/eLife.87181. Elife. 2023. PMID: 37428017 Free PMC article.
-
Rho of Plants patterning: linking mathematical models and molecular diversity.J Exp Bot. 2024 Feb 28;75(5):1274-1288. doi: 10.1093/jxb/erad447. J Exp Bot. 2024. PMID: 37962515 Free PMC article. Review.
-
Spatial models of pattern formation during phagocytosis.PLoS Comput Biol. 2022 Oct 3;18(10):e1010092. doi: 10.1371/journal.pcbi.1010092. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36190993 Free PMC article.
-
Membrane Tension Can Enhance Adaptation to Maintain Polarity of Migrating Cells.Biophys J. 2020 Oct 20;119(8):1617-1629. doi: 10.1016/j.bpj.2020.08.035. Epub 2020 Sep 7. Biophys J. 2020. PMID: 32976760 Free PMC article.
-
On the influence of cell shape on dynamic reaction-diffusion polarization patterns.PLoS One. 2021 Mar 18;16(3):e0248293. doi: 10.1371/journal.pone.0248293. eCollection 2021. PLoS One. 2021. PMID: 33735291 Free PMC article.
References
-
- Alnaes MS, Blechta J, Hake J, Johansson A, Kehlet B, Logg A, Richardson C, Ring J, Rognes ME, Wells GN. The FEniCS project version 1.5. Arch Numer Softw. 2015;3(100):9–23.
-
- Buttenschön A, Liu Y, Edelstein-Keshet L. Cell size, mechanical tension, and GTPase signaling in the single cell. Bull Math Biol. 2019;82(2):1–33. - PubMed
-
- Champneys AR, Al Saadi F, Breña -Medina VF, Grieneisen VA, Marée AFM, Verschueren N, Wuyts B (2021) Bistability, wave pinning and localisation in natural reaction-diffusion systems. Phys D: Nonlinear Phenom 416(132735). 10.1016/j.physd.2020.132735
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

