WW domain-binding protein 2 overexpression prevents diet-induced liver steatosis and insulin resistance through AMPKβ1
- PMID: 33658485
- PMCID: PMC7930037
- DOI: 10.1038/s41419-021-03536-8
WW domain-binding protein 2 overexpression prevents diet-induced liver steatosis and insulin resistance through AMPKβ1
Abstract
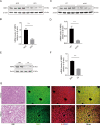
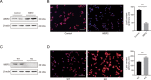
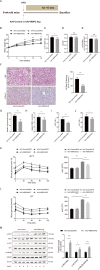
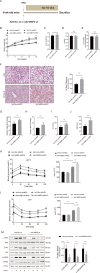
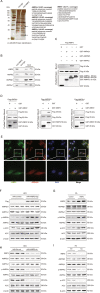
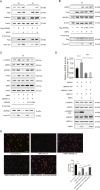
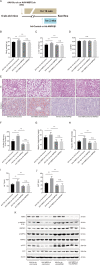
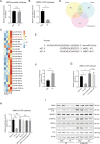
Nonalcoholic fatty liver disease (NAFLD) is prevalent clinically and can lead to more serious chronic liver disease. However, the pathological mechanism is still unclear, and thus, there are no approved drugs on the market. Transcriptional coactivator WW domain-binding protein 2 (WBP2) is a newly discovered oncogene that has an important relationship with the occurrence and development of breast cancer and mediates the interaction between Wnt and various other signaling pathways. The expression level of WBP2 was decreased in NAFLD. Overexpression of WBP2 with AAV in vivo alleviated liver fat deposition and insulin resistance induced by a high-fat diet (HFD). Knockdown of WBP2 with AAV aggravated HFD-induced fatty liver and insulin resistance. In vitro experiments showed that in the human normal hepatocyte cell line LO2 and primary hepatocytes isolated from mice, overexpression of WBP2 reduced fat deposition, and knocking out or knocking down WBP2 aggravated PA-induced fat deposition. Through mass spectrometry, we found that WBP2 can bind to AMPKβ1, and by mutating AMPKβ1, we found that WBP2 can induce phosphorylation of AMPKβ1 at S108 and then activate the AMPK pathway to affect lipid metabolism. The effect of WBP2 on NAFLD provides a possible new direction for future research on NAFLD.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
GCSF deficiency attenuates nonalcoholic fatty liver disease through regulating GCSFR-SOCS3-JAK-STAT3 pathway and immune cells infiltration.Am J Physiol Gastrointest Liver Physiol. 2021 Apr 1;320(4):G531-G542. doi: 10.1152/ajpgi.00342.2020. Epub 2021 Jan 20. Am J Physiol Gastrointest Liver Physiol. 2021. PMID: 33470903
-
Degradation of PHLPP2 by KCTD17, via a Glucagon-Dependent Pathway, Promotes Hepatic Steatosis.Gastroenterology. 2017 Dec;153(6):1568-1580.e10. doi: 10.1053/j.gastro.2017.08.039. Epub 2017 Aug 30. Gastroenterology. 2017. PMID: 28859855 Free PMC article.
-
IGFBP5 modulates lipid metabolism and insulin sensitivity through activating AMPK pathway in non-alcoholic fatty liver disease.Life Sci. 2020 Sep 1;256:117997. doi: 10.1016/j.lfs.2020.117997. Epub 2020 Jun 22. Life Sci. 2020. PMID: 32585242
-
Circ_0057558 promotes nonalcoholic fatty liver disease by regulating ROCK1/AMPK signaling through targeting miR-206.Cell Death Dis. 2021 Aug 26;12(9):809. doi: 10.1038/s41419-021-04090-z. Cell Death Dis. 2021. PMID: 34446693 Free PMC article.
-
The emerging roles of WBP2 oncogene in human cancers.Oncogene. 2020 Jun;39(24):4621-4635. doi: 10.1038/s41388-020-1318-0. Epub 2020 May 11. Oncogene. 2020. PMID: 32393834 Free PMC article. Review.
Cited by
-
Hepatic estrogen receptor alpha drives masculinization in post-menopausal women with metabolic dysfunction-associated steatotic liver disease.JHEP Rep. 2024 Jun 13;6(10):101143. doi: 10.1016/j.jhepr.2024.101143. eCollection 2024 Oct. JHEP Rep. 2024. PMID: 39308985 Free PMC article.
-
WW domain binding protein 2 (WBP2) as an oncogene in breast cancer: mechanisms and therapeutic prospects-a narrative review.Gland Surg. 2022 Dec;11(12):1984-2002. doi: 10.21037/gs-22-716. Gland Surg. 2022. PMID: 36654949 Free PMC article. Review.
-
Signaling mechanisms in renal compensatory hypertrophy revealed by multi-omics.Nat Commun. 2023 Jun 16;14(1):3481. doi: 10.1038/s41467-023-38958-9. Nat Commun. 2023. PMID: 37328470 Free PMC article.
-
Phosphorylation: new star of pathogenesis and treatment in steatotic liver disease.Lipids Health Dis. 2024 Feb 17;23(1):50. doi: 10.1186/s12944-024-02037-9. Lipids Health Dis. 2024. PMID: 38368351 Free PMC article.
-
Screening of co-pathogenic genes of non-alcoholic fatty liver disease and hepatocellular carcinoma.Front Oncol. 2022 Aug 11;12:911808. doi: 10.3389/fonc.2022.911808. eCollection 2022. Front Oncol. 2022. PMID: 36033523 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

