Anti-EGFR VHH-armed death receptor ligand-engineered allogeneic stem cells have therapeutic efficacy in diverse brain metastatic breast cancers
- PMID: 33658202
- PMCID: PMC7929513
- DOI: 10.1126/sciadv.abe8671
Anti-EGFR VHH-armed death receptor ligand-engineered allogeneic stem cells have therapeutic efficacy in diverse brain metastatic breast cancers
Abstract
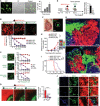
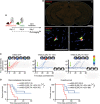
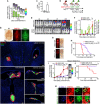
Basal-like breast cancer (BLBC) shows brain metastatic (BM) capability and overexpresses EGFR and death-receptors 4/5 (DR4/5); however, the anatomical location of BM prohibits efficient drug-delivery to these targetable markers. In this study, we developed BLBC-BM mouse models featuring different patterns of BMs and explored the versatility of estem cell (SC)-mediated bi-functional EGFR and DR4/5-targeted treatment in these models. Most BLBC lines demonstrated a high sensitivity to EGFR and DR4/5 bi-targeting therapeutic protein, EVDRL [anti-EGFR VHH (EV) fused to DR ligand (DRL)]. Functional analyses using inhibitors and CRISPR-Cas9 knockouts revealed that the EV domain facilitated in augmenting DR4/5-DRL binding and enhancing DRL-induced apoptosis. EVDRL secreting stem cells alleviated tumor-burden and significantly increased survival in mouse models of residual-tumor after macrometastasis resection, perivascular niche micrometastasis, and leptomeningeal metastasis. This study reports mechanism based simultaneous targeting of EGFR and DR4/5 in BLBC and defines a new treatment paradigm for treatment of BM.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures




Similar articles
-
Fate and Efficacy of Engineered Allogeneic Stem Cells Targeting Cell Death and Proliferation Pathways in Primary and Brain Metastatic Lung Cancer.Stem Cells Transl Med. 2023 Jul 14;12(7):444-458. doi: 10.1093/stcltm/szad033. Stem Cells Transl Med. 2023. PMID: 37311043 Free PMC article.
-
Targeting breast to brain metastatic tumours with death receptor ligand expressing therapeutic stem cells.Brain. 2015 Jun;138(Pt 6):1710-21. doi: 10.1093/brain/awv094. Epub 2015 Apr 23. Brain. 2015. PMID: 25910782 Free PMC article.
-
Tumorigenicity of EGFR- and/or HER2-Positive Breast Cancers Is Mediated by Recruitment of Tumor-Associated Macrophages.Int J Mol Sci. 2023 Jan 11;24(2):1443. doi: 10.3390/ijms24021443. Int J Mol Sci. 2023. PMID: 36674955 Free PMC article.
-
Epidermal growth factor receptor as a therapeutic target in glioblastoma.Neuromolecular Med. 2013 Jun;15(2):420-34. doi: 10.1007/s12017-013-8229-y. Epub 2013 Apr 11. Neuromolecular Med. 2013. PMID: 23575987 Review.
-
Small molecule inhibitors targeting the EGFR/ErbB family of protein-tyrosine kinases in human cancers.Pharmacol Res. 2019 Jan;139:395-411. doi: 10.1016/j.phrs.2018.11.014. Epub 2018 Nov 27. Pharmacol Res. 2019. PMID: 30500458 Review.
Cited by
-
Mechanotransduction through adhesion molecules: Emerging roles in regulating the stem cell niche.Front Cell Dev Biol. 2022 Sep 12;10:966662. doi: 10.3389/fcell.2022.966662. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36172276 Free PMC article. Review.
-
Targeted drug delivery using nanobodies to deliver effective molecules to breast cancer cells: the most attractive application of nanobodies.Cancer Cell Int. 2024 Feb 10;24(1):67. doi: 10.1186/s12935-024-03259-8. Cancer Cell Int. 2024. PMID: 38341580 Free PMC article. Review.
-
Single domain Camelid antibody fragments for molecular imaging and therapy of cancer.Front Oncol. 2023 Sep 8;13:1257175. doi: 10.3389/fonc.2023.1257175. eCollection 2023. Front Oncol. 2023. PMID: 37746282 Free PMC article. Review.
-
Allogeneic stem cells engineered to release interferon β and scFv-PD1 target glioblastoma and alter the tumor microenvironment.Cytotherapy. 2024 Oct;26(10):1217-1226. doi: 10.1016/j.jcyt.2024.05.012. Epub 2024 May 17. Cytotherapy. 2024. PMID: 38852095
-
Development of microsurgical forceps equipped with haptic technology for in situ differentiation of brain tumors during microsurgery.Sci Rep. 2024 Sep 13;14(1):21430. doi: 10.1038/s41598-024-72326-x. Sci Rep. 2024. PMID: 39271763 Free PMC article.
References
-
- Tabouret E., Chinot O., Metellus P., Tallet A., Viens P., Gonçalves A., Recent trends in epidemiology of brain metastases: An overview. Anticancer Res 32, 4655–4662 (2012). - PubMed
-
- Lin N. U., Bellon J. R., Winer E. P., CNS metastases in breast cancer. J. Clin. Oncol. 22, 3608–3617 (2004). - PubMed
-
- Siegel R. L., Miller K. D., Jemal A., Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 (2018). - PubMed
-
- Mack F., Baumert B. G., Schäfer N., Hattingen E., Scheffler B., Herrlinger U., Glas M., Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat. Rev. 43, 83–91 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

