New cell delivery system CellSaic with adipose-derived stromal cells promotes functional angiogenesis in critical limb ischemia model mice
- PMID: 33656644
- PMCID: PMC8380570
- DOI: 10.1007/s10047-021-01254-8
New cell delivery system CellSaic with adipose-derived stromal cells promotes functional angiogenesis in critical limb ischemia model mice
Abstract
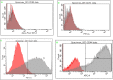
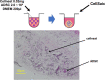
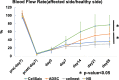
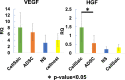
Current therapies for patients with critical limb ischemia have not reduced amputation risk owing to poor cell engraftment. The recombinant peptide Cellnest increases the engraftment rate of administered cells by forming a complex with the cells (CellSaic). We hypothesized that CellSaic containing adipose-derived stromal cells (ADSCs) could improve lower limb blood flow better than ADSCs alone, resulting in better transplanted cell engraftment. ADSCs were extracted from 8-week-old C57BL/6N mice. Thirty-two critical limb ischemia model mice were established by ligating femoral arteries. They were divided into CellSaic (n = 11), ADSC (n = 10), saline (n = 9), and Cellnest (n = 9) groups. Blood flow rate (affected side blood flow / healthy side blood flow × 100%) was evaluated using a laser Doppler blood flow meter every week. Mice were euthanized on day 28 for histological evaluation. Compared with the ADSC group (54.5 ± 17.2%), treated side blood flow rate of the CellSaic group (78.0 ± 24.9%) showed significant improvement on day 28 after administration (p < 0.05). CD31 staining showed significantly higher number of capillary vessels in the CellSaic group (53.0 ± 8.9 cells/mm3) than in the ADSC group (43.0 ± 6.8 cells/mm3) (p < 0.05). Fluorescent staining showed significantly higher number of arterioles containing both CD31 and αSMA double-positive cells in the CellSaic group than in the ADSC group (p < 0.05). CellSaic containing ADSCs exhibited superiority to ADSC transplantation alone in promoting functional angiogenesis, suggesting its potential in improving clinical outcomes of angiogenic therapy for ischemic limbs.
Keywords: Adipose-derived stromal cell; Angiogenesis; Critical limb ischemia; Engraftment rate.
© 2021. The Author(s).
Conflict of interest statement
We know of no conflicts of interest associated with this publication, and there has been no significant financial support for this work that could have influenced its outcome.
Figures


Similar articles
-
Low-molecular weight heparin protamine complex augmented the potential of adipose-derived stromal cells to ameliorate limb ischemia.Atherosclerosis. 2016 Jun;249:132-9. doi: 10.1016/j.atherosclerosis.2016.04.003. Epub 2016 Apr 6. Atherosclerosis. 2016. PMID: 27100923
-
The Therapeutic Effects of Adipose-Derived Stem Cells and Recombinant Peptide Pieces on Mouse Model of DSS Colitis.Cell Transplant. 2018 Sep;27(9):1390-1400. doi: 10.1177/0963689718782442. Epub 2018 Jul 6. Cell Transplant. 2018. PMID: 29978718 Free PMC article.
-
Glyoxalase-1 Overexpression Reverses Defective Proangiogenic Function of Diabetic Adipose-Derived Stem Cells in Streptozotocin-Induced Diabetic Mice Model of Critical Limb Ischemia.Stem Cells Transl Med. 2017 Jan;6(1):261-271. doi: 10.5966/sctm.2015-0380. Epub 2016 Aug 15. Stem Cells Transl Med. 2017. PMID: 28170200 Free PMC article.
-
Exosomes derived from adipose-derived stem cells overexpressing glyoxalase-1 protect endothelial cells and enhance angiogenesis in type 2 diabetic mice with limb ischemia.Stem Cell Res Ther. 2021 Jul 15;12(1):403. doi: 10.1186/s13287-021-02475-7. Stem Cell Res Ther. 2021. PMID: 34266474 Free PMC article.
-
Adipose-Derived Stromal Cell-Based Therapies for Radiation-Induced Fibrosis.Adv Wound Care (New Rochelle). 2024 May;13(5):235-252. doi: 10.1089/wound.2022.0103. Epub 2022 Dec 9. Adv Wound Care (New Rochelle). 2024. PMID: 36345216 Review.
Cited by
-
Evaluation of Osteogenic Potential for Rat Adipose-Derived Stem Cells under Xeno-Free Environment.Int J Mol Sci. 2023 Dec 15;24(24):17532. doi: 10.3390/ijms242417532. Int J Mol Sci. 2023. PMID: 38139360 Free PMC article.
References
-
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR, TASC II Working Group. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45:S5–67. - PubMed
-
- Nakagami H, Maeda K, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue–derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. - DOI - PubMed
-
- Kapp TG, Rechenmacher F, Neubauer S, Maltsev OV, Cavalcanti-Adam EA, Zarka R, Reuning U, Notni J, Wester HJ, Mas-Moruno C, Spatz J, Geiger B, Kessler H. A comprehensive evaluation of the activity and selectivity profile of ligands for RGD-binding integrins. Sci Rep. 2017 doi: 10.1038/srep39805. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

