Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery
- PMID: 33653317
- PMCID: PMC7923597
- DOI: 10.1186/s12885-021-07925-2
Phase II trial of prophylactic hyperthermic intraperitoneal chemotherapy in patients with locally advanced gastric cancer after curative surgery
Abstract
Background: HIPEC is an emerging procedure to treat peritoneal metastasis of gastric cancer. Data about HIPEC in locally advanced gastric cancer is scarce. The purpose of this trial is to evaluate the safety and toxicity of prophylactic HIPEC with cisplatin for patients with locally advanced gastric cancer.
Methods: From March 2015 to November 2016, a prospective, randomized phase II trial was conducted. After radical gastrectomy, patients in the experimental group underwent HIPEC with cisplatin followed by adjuvant chemotherapy with SOX regime. Patients in the other group were treated with SOX regime alone. Postoperative complications and patient survival were compared.
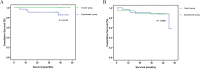
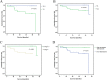
Results: In total, 50 patients were eligible for analyses. No significant difference was found in the incidence of postoperative complications including anastomotic/intestinal leakage, liver dysfunction, bone marrow suppression, wound infection and ileus (P > 0.05). Mean duration of hospitalization after radical gastrectomy was 11.7 days. 12.2 days in experimental group and 10.8 days in control group respectively (P = 0.255). The percentage of patients with elevated tumor markers was 12.1% in experimental group, which was significantly lower than 41.2% in control group (P = 0.02). 3-year RFS of patients who treated with or without prophylactic HIPEC were 84.8 and 88.2% respectively (P = 0.986). In the multivariate analysis, pathological T stage was the only independent risk factor for the RFS of patients (P = 0.012, HR =15.071).
Conclusion: Additional intraoperative HIPEC with cisplatin did not increase postoperative complications for locally advanced gastric cancer after curative surgery. Prophylactic HIPEC with cisplatin was safe and tolerable, while it did not reduce the risk of peritoneal recurrence in this trial, supporting further studies to validate the efficacy of it.
Trial registration: Chinese Clinical Trial Registry, ChiCTR2000038331. Registered 18 September 2020 - Retrospectively registered, http://www.chictr.org.cn/showproj.aspx?proj=59692 .
Keywords: Cisplatin; Clinical efficacy; HIPEC; Locally advanced gastric cancer; Safety.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Efficacy of neoadjuvant chemotherapy combined with prophylactic intraperitoneal hyperthermic chemotherapy for patients diagnosed with clinical T4 gastric cancer who underwent laparoscopic radical gastrectomy: a retrospective cohort study based on propensity score matching.World J Surg Oncol. 2024 Sep 11;22(1):244. doi: 10.1186/s12957-024-03526-y. World J Surg Oncol. 2024. PMID: 39256787 Free PMC article.
-
[Safety evaluation of hyperthermic intraperitoneal chemotherapy in patients with local advanced gastric cancer after radical resection for prevention of peritoneal metastasis].Zhonghua Wei Chang Wai Ke Za Zhi. 2022 Jan 25;25(1):48-55. doi: 10.3760/cma.j.cn441530-20210514-00206. Zhonghua Wei Chang Wai Ke Za Zhi. 2022. PMID: 35067034 Chinese.
-
Prophylactic HIPEC with radical D2 gastrectomy improves survival and peritoneal recurrence rates for locally advanced gastric cancer: personal experience from a randomized case control study.BMC Cancer. 2019 Sep 18;19(1):932. doi: 10.1186/s12885-019-6125-z. BMC Cancer. 2019. PMID: 31533660 Free PMC article.
-
[Progress in prophylatic hyperthermic intraperitoneal chemotherapy for advanced gastric carcinoma].Zhonghua Wei Chang Wai Ke Za Zhi. 2018 May 25;21(5):593-599. Zhonghua Wei Chang Wai Ke Za Zhi. 2018. PMID: 29774943 Review. Chinese.
-
The 30-year experience-A meta-analysis of randomised and high-quality non-randomised studies of hyperthermic intraperitoneal chemotherapy in the treatment of gastric cancer.Eur J Cancer. 2017 Jul;79:1-14. doi: 10.1016/j.ejca.2017.03.030. Epub 2017 Apr 26. Eur J Cancer. 2017. PMID: 28456089 Free PMC article. Review.
Cited by
-
Hyperthermic intraperitoneal chemotherapy for management of gastrointestinal and biliary tract malignancies: a systematic review and meta-analysis of randomized trials.Ann Gastroenterol. 2023 Jan-Feb;36(1):87-96. doi: 10.20524/aog.2023.0758. Epub 2022 Nov 15. Ann Gastroenterol. 2023. PMID: 36593815 Free PMC article.
-
Efficacy of neoadjuvant chemotherapy combined with prophylactic intraperitoneal hyperthermic chemotherapy for patients diagnosed with clinical T4 gastric cancer who underwent laparoscopic radical gastrectomy: a retrospective cohort study based on propensity score matching.World J Surg Oncol. 2024 Sep 11;22(1):244. doi: 10.1186/s12957-024-03526-y. World J Surg Oncol. 2024. PMID: 39256787 Free PMC article.
-
Hyperthermic Intraperitoneal Chemotherapy (HIPEC) Combined with Surgery: A 12-Year Meta-Analysis of this Promising Treatment Strategy for Advanced Gastric Cancer at Different Stages.Ann Surg Oncol. 2022 May;29(5):3170-3186. doi: 10.1245/s10434-021-11316-z. Epub 2022 Feb 17. Ann Surg Oncol. 2022. PMID: 35175455 Review.
-
Hyperthermic Intraperitoneal Chemotherapy in the Management of Gastric Cancer: A Narrative Review.Int J Environ Res Public Health. 2022 Jan 7;19(2):681. doi: 10.3390/ijerph19020681. Int J Environ Res Public Health. 2022. PMID: 35055500 Free PMC article. Review.
-
The short- and long-term survival of hyperthermic intraperitoneal chemotherapy (HIPEC) in the advanced gastric cancer with/without peritoneal carcinomatosis: a systematic review and meta-analysis of randomized controlled trials.Updates Surg. 2022 Dec;74(6):1805-1816. doi: 10.1007/s13304-022-01376-5. Epub 2022 Sep 18. Updates Surg. 2022. PMID: 36116077 Review.
References
-
- Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–363. doi: 10.1002/(SICI)1097-0142(20000115)88:2<358::AID-CNCR16>3.0.CO;2-O. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous