Differential Responses to Bioink-Induced Oxidative Stress in Endothelial Cells and Fibroblasts
- PMID: 33652991
- PMCID: PMC7956320
- DOI: 10.3390/ijms22052358
Differential Responses to Bioink-Induced Oxidative Stress in Endothelial Cells and Fibroblasts
Abstract
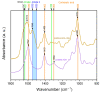
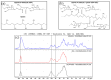
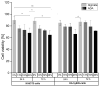
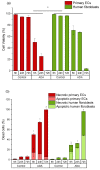
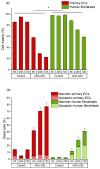
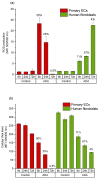
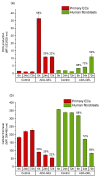
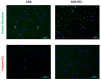
A hydrogel system based on oxidized alginate covalently crosslinked with gelatin (ADA-GEL) has been utilized for different biofabrication approaches to design constructs, in which cell growth, proliferation and migration have been observed. However, cell-bioink interactions are not completely understood and the potential effects of free aldehyde groups on the living cells have not been investigated. In this study, alginate, ADA and ADA-GEL were characterized via FTIR and NMR, and their effect on cell viability was investigated. In the tested cell lines, there was a concentration-dependent effect of oxidation degree on cell viability, with the strongest cytotoxicity observed after 72 h of culture. Subsequently, primary human cells, namely fibroblasts and endothelial cells (ECs) were grown in ADA and ADA-GEL hydrogels to investigate the molecular effects of oxidized material. In ADA, an extremely strong ROS generation resulting in a rapid depletion of cellular thiols was observed in ECs, leading to rapid necrotic cell death. In contrast, less pronounced cytotoxic effects of ADA were noted on human fibroblasts. Human fibroblasts had higher cellular thiol content than primary ECs and entered apoptosis under strong oxidative stress. The presence of gelatin in the hydrogel improved the primary cell survival, likely by reducing the oxidative stress via binding to the CHO groups. Consequently, ADA-GEL was better tolerated than ADA alone. Fibroblasts were able to survive the oxidative stress in ADA-GEL and re-entered the proliferative phase. To the best of our knowledge, this is the first report that shows in detail the relationship between oxidative stress-induced intracellular processes and alginate di-aldehyde-based bioinks.
Keywords: alginate di-aldehyde; cell death; cell viability; gelatin; glutathione; oxidative stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Evaluation of fibroblasts adhesion and proliferation on alginate-gelatin crosslinked hydrogel.PLoS One. 2014 Sep 30;9(9):e107952. doi: 10.1371/journal.pone.0107952. eCollection 2014. PLoS One. 2014. PMID: 25268892 Free PMC article.
-
Biofabrication of vessel-like structures with alginate di-aldehyde-gelatin (ADA-GEL) bioink.J Mater Sci Mater Med. 2018 Dec 29;30(1):8. doi: 10.1007/s10856-018-6205-7. J Mater Sci Mater Med. 2018. PMID: 30594988
-
Composite Alginate Dialdehyde-Gelatin (ADA-GEL) Hydrogel Containing Short Ribbon-Shaped Fillers for Skeletal Muscle Tissue Biofabrication.ACS Appl Mater Interfaces. 2024 Aug 28;16(34):44605-44622. doi: 10.1021/acsami.4c10751. Epub 2024 Aug 19. ACS Appl Mater Interfaces. 2024. PMID: 39159061
-
Cell specificity of magnetic cell seeding approach to hydrogel colonization.J Biomed Mater Res A. 2017 Nov;105(11):2948-2957. doi: 10.1002/jbm.a.36147. Epub 2017 Jul 14. J Biomed Mater Res A. 2017. PMID: 28639348
-
A review on alginate-based bioinks, combination with other natural biomaterials and characteristics.J Biomater Appl. 2022 Aug;37(2):355-372. doi: 10.1177/08853282221085690. Epub 2022 May 5. J Biomater Appl. 2022. PMID: 35510845 Review.
Cited by
-
Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models.Pharmaceuticals (Basel). 2022 Oct 27;15(11):1328. doi: 10.3390/ph15111328. Pharmaceuticals (Basel). 2022. PMID: 36355501 Free PMC article.
-
Layer-by-Layer Cell Encapsulation for Drug Delivery: The History, Technique Basis, and Applications.Pharmaceutics. 2022 Jan 27;14(2):297. doi: 10.3390/pharmaceutics14020297. Pharmaceutics. 2022. PMID: 35214030 Free PMC article. Review.
-
Functionalized Gelatin/Polysaccharide Hydrogels for Encapsulation of Hepatocytes.Gels. 2024 Mar 28;10(4):231. doi: 10.3390/gels10040231. Gels. 2024. PMID: 38667650 Free PMC article.
-
Endothelialization of Whey Protein Isolate-Based Scaffolds for Tissue Regeneration.Molecules. 2023 Oct 12;28(20):7052. doi: 10.3390/molecules28207052. Molecules. 2023. PMID: 37894531 Free PMC article.
-
Engineering peptide-modified alginate-based bioinks with cell-adhesive properties for biofabrication.RSC Adv. 2024 Apr 26;14(20):13769-13786. doi: 10.1039/d3ra08394b. eCollection 2024 Apr 25. RSC Adv. 2024. PMID: 38681843 Free PMC article.
References
-
- Li H.J., Tan C., Li L. Review of 3D printable hydrogels and constructs. Mater. Des. 2018;159:20–38. doi: 10.1016/j.matdes.2018.08.023. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

