Transcriptome profiling of Lymnaea stagnalis (Gastropoda) for ecoimmunological research
- PMID: 33648459
- PMCID: PMC7919325
- DOI: 10.1186/s12864-021-07428-1
Transcriptome profiling of Lymnaea stagnalis (Gastropoda) for ecoimmunological research
Abstract
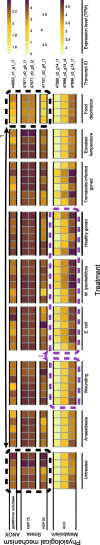
Background: Host immune function can contribute to numerous ecological/evolutionary processes. Ecoimmunological studies, however, typically use one/few phenotypic immune assays and thus do not consider the complexity of the immune system. Therefore, "omics" resources that allow quantifying immune activity across multiple pathways are needed for ecoimmunological models. We applied short-read based RNAseq (Illumina NextSeq 500, PE-81) to characterise transcriptome profiles of Lymnaea stagnalis (Gastropoda), a multipurpose model snail species. We used a genetically diverse snail stock and exposed individuals to immune elicitors (injury, bacterial/trematode pathogens) and changes in environmental conditions that can alter immune activity (temperature, food availability).
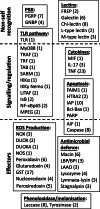
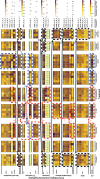
Results: Immune defence factors identified in the de novo assembly covered elements broadly described in other gastropods. For instance, pathogen-recognition receptors (PRR) and lectins activate Toll-like receptor (TLR) pathway and cytokines that regulate cellular and humoral defences. Surprisingly, only modest diversity of antimicrobial peptides and fibrinogen related proteins were detected when compared with other taxa. Additionally, multiple defence factors that may contribute to the phenotypic immune assays used to quantify antibacterial activity and phenoloxidase (PO)/melanisation-type reaction in this species were found. Experimental treatments revealed factors from non-self recognition (lectins) and signalling (TLR pathway, cytokines) to effectors (e.g., antibacterial proteins, PO enzymes) whose transcription depended on immune stimuli and environmental conditions, as well as components of snail physiology/metabolism that may drive these effects. Interestingly, the transcription of many factors (e.g., PRR, lectins, cytokines, PO enzymes, antibacterial proteins) showed high among-individual variation.
Conclusions: Our results indicate several uniform aspects of gastropod immunity, but also apparent differences between L. stagnalis and some previously examined taxa. Interestingly, in addition to immune defence factors that responded to immune elicitors and changes in environmental conditions, many factors showed high among-individual variation across experimental snails. We propose that such factors are highly important to be included in future ecoimmunological studies because they may be the key determinants of differences in parasite resistance among individuals both within and between natural snail populations.
Keywords: Ecological immunology; Great pond snail; Mollusc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Activation of the immune defence of the freshwater snail Lymnaea stagnalis by different immune elicitors.J Exp Biol. 2013 Aug 1;216(Pt 15):2902-7. doi: 10.1242/jeb.084947. J Exp Biol. 2013. PMID: 23842628
-
Ion channel profiling of the Lymnaea stagnalis ganglia via transcriptome analysis.BMC Genomics. 2021 Jan 6;22(1):18. doi: 10.1186/s12864-020-07287-2. BMC Genomics. 2021. PMID: 33407100 Free PMC article.
-
Maintenance of genetic variation in immune defense of a freshwater snail: role of environmental heterogeneity.Evolution. 2010 Aug;64(8):2397-407. doi: 10.1111/j.1558-5646.2010.00995.x. Epub 2010 Mar 18. Evolution. 2010. PMID: 20298461
-
Examining adaptive evolution of immune activity: opportunities provided by gastropods in the age of 'omics'.Philos Trans R Soc Lond B Biol Sci. 2021 May 24;376(1825):20200158. doi: 10.1098/rstb.2020.0158. Epub 2021 Apr 5. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33813886 Free PMC article. Review.
-
Snail defence responses to parasite infection: The Lymnaea stagnalis-Trichobilharzia szidati model.Dev Comp Immunol. 2020 Jan;102:103464. doi: 10.1016/j.dci.2019.103464. Epub 2019 Aug 8. Dev Comp Immunol. 2020. PMID: 31402190 Review.
Cited by
-
LPS-Induced Garcia Effect and Its Pharmacological Regulation Mediated by Acetylsalicylic Acid: Behavioral and Transcriptional Evidence.Biology (Basel). 2023 Aug 7;12(8):1100. doi: 10.3390/biology12081100. Biology (Basel). 2023. PMID: 37626986 Free PMC article.
-
Integration of Transcriptomics and Microbiomics Reveals the Responses of Bellamya aeruginosa to Toxic Cyanobacteria.Toxins (Basel). 2023 Feb 1;15(2):119. doi: 10.3390/toxins15020119. Toxins (Basel). 2023. PMID: 36828433 Free PMC article.
-
In search of the Aplysia immunome: an in silico study.BMC Genomics. 2022 Jul 29;23(1):543. doi: 10.1186/s12864-022-08780-6. BMC Genomics. 2022. PMID: 35906538 Free PMC article.
-
Transcriptome analysis provides genome annotation and expression profiles in the central nervous system of Lymnaea stagnalis at different ages.BMC Genomics. 2021 Sep 3;22(1):637. doi: 10.1186/s12864-021-07946-y. BMC Genomics. 2021. PMID: 34479505 Free PMC article.
-
Yolk proteins of the schistosomiasis vector snail Biomphalaria glabrata revealed by multi-omics analysis.Sci Rep. 2024 Jan 20;14(1):1820. doi: 10.1038/s41598-024-52392-x. Sci Rep. 2024. PMID: 38245605 Free PMC article.
References
-
- Schmid-Hempel P. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. New York: Oxford University Press; 2011.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

