EGFR mutation-guided use of afatinib, erlotinib and gefitinib for advanced non-small-cell lung cancer in Hong Kong - A cost-effectiveness analysis
- PMID: 33647045
- PMCID: PMC7920377
- DOI: 10.1371/journal.pone.0247860
EGFR mutation-guided use of afatinib, erlotinib and gefitinib for advanced non-small-cell lung cancer in Hong Kong - A cost-effectiveness analysis
Abstract
Introduction: Tyrosine kinase inhibitors (TKIs) therapy targets at epidermal growth factor receptor (EGFR) gene mutations in non-small-cell lung cancer (NSCLC). We aimed to compare the EGFR mutation-guided target therapy versus empirical chemotherapy for first-line treatment of advanced NSCLC in the public healthcare setting of Hong Kong.
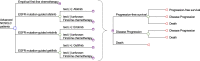
Methods: A Markov model was designed to simulate outcomes of a hypothetical cohort of advanced (stage IIIB/IV) NSCLC adult patients with un-tested EGFR-sensitizing mutation status. Four treatment strategies were evaluated: Empirical first-line chemotherapy with cisplatin-pemetrexed (empirical chemotherapy group), and EGFR mutation-guided use of a TKI (afatinib, erlotinib, and gefitinib). Model outcome measures were direct medical cost, progression-free survival, overall survival, and quality-adjusted life-years (QALYs). Incremental cost per QALY gained (ICER) was estimated. Sensitivity analyses were performed to examine robustness of model results.
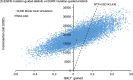
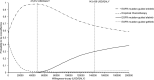
Results: Empirical chemotherapy and EGFR mutation-guided gefitinib gained lower QALYs at higher costs than the erlotinib group. Comparing with EGFR mutation-guided erlotinib, the afatinib strategy gained additional QALYs with ICER (540,633 USD/QALY). In 10,000 Monte Carlo simulations for probabilistic sensitivity analysis, EGFR mutation-guided afatinib, erlotinib, gefitinib and empirical chemotherapy were preferred strategy in 0%, 98%, 0% and 2% of time at willingness-to-pay (WTP) 47,812 USD/QALY (1x gross domestic product (GDP) per capita), and in 30%, 68%, 2% and 0% of time at WTP 143,436 USD/QALY (3x GDP per capita), respectively.
Conclusions: EGFR mutation-guided erlotinib appears to be the cost-effective strategy from the perspective of Hong Kong public healthcare provider over a broad range of WTP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Cost-Effectiveness and Value of Information of Erlotinib, Afatinib, and Cisplatin-Pemetrexed for First-Line Treatment of Advanced EGFR Mutation-Positive Non-Small-Cell Lung Cancer in the United States.Value Health. 2015 Sep;18(6):774-82. doi: 10.1016/j.jval.2015.04.008. Epub 2015 Jun 22. Value Health. 2015. PMID: 26409604
-
Cost-effectiveness of afatinib, gefitinib, erlotinib and pemetrexed-based chemotherapy as first-line treatments for advanced non-small cell lung cancer in China.Lung Cancer. 2019 Jan;127:84-89. doi: 10.1016/j.lungcan.2018.11.029. Epub 2018 Nov 24. Lung Cancer. 2019. PMID: 30642557
-
Cost-effectiveness of Osimertinib in the First-Line Treatment of Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer.JAMA Oncol. 2018 Aug 1;4(8):1080-1084. doi: 10.1001/jamaoncol.2018.1395. JAMA Oncol. 2018. PMID: 29852038 Free PMC article. Clinical Trial.
-
First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer.Cochrane Database Syst Rev. 2016 May 25;(5):CD010383. doi: 10.1002/14651858.CD010383.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2021 Mar 18;3:CD010383. doi: 10.1002/14651858.CD010383.pub3. PMID: 27223332 Updated. Review.
-
Gefitinib for the first-line treatment of locally advanced or metastatic non-small cell lung cancer.Health Technol Assess. 2010 Oct;14(Suppl. 2):71-9. doi: 10.3310/hta14suppl2/10. Health Technol Assess. 2010. PMID: 21047494 Review.
Cited by
-
Maximizing the cost-effectiveness of cervical screening in the context of routine HPV vaccination by optimizing screening strategies with respect to vaccine uptake: a modeling analysis.BMC Med. 2023 Feb 10;21(1):48. doi: 10.1186/s12916-023-02748-3. BMC Med. 2023. PMID: 36765349 Free PMC article.
-
Critical Examination of Modeling Approaches Used in Economic Evaluations of First-Line Treatments for Locally Advanced or Metastatic Non-Small Cell Lung Cancer Harboring Epidermal Growth Factor Receptor Mutations: A Systematic Literature Review.Pharmacoeconomics. 2024 May;42(5):527-568. doi: 10.1007/s40273-024-01362-2. Epub 2024 Mar 15. Pharmacoeconomics. 2024. PMID: 38489077 Free PMC article.
-
QALY-type preference and willingness-to-pay among end-of-life patients with cancer treatments: a pilot study using discrete choice experiment.Qual Life Res. 2024 Mar;33(3):753-765. doi: 10.1007/s11136-023-03562-3. Epub 2023 Dec 11. Qual Life Res. 2024. PMID: 38079024
-
Comparison of T790M Acquisition After Treatment With First- and Second-Generation Tyrosine-Kinase Inhibitors: A Systematic Review and Network Meta-Analysis.Front Oncol. 2022 Jun 28;12:869390. doi: 10.3389/fonc.2022.869390. eCollection 2022. Front Oncol. 2022. PMID: 35837103 Free PMC article.
-
Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis.World J Clin Cases. 2022 Jul 6;10(19):6360-6369. doi: 10.12998/wjcc.v10.i19.6360. World J Clin Cases. 2022. PMID: 35979322 Free PMC article. Review.
References
-
- Hospital Authority. Overview of Hong Kong cancer statistics of 2017. [cited 20 April 2020]. Available from: https://www3.ha.org.hk/cancereg/pdf/overview/Summary%20of%20CanStat%2020...
-
- Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, et al.. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62. 10.1097/JTO.0000000000000033 - DOI - PMC - PubMed
-
- National Comprehensive Cancer Network Clinical Practice Guideline in Oncology (NCCN Guidelines) Non-small cell lung cancer. Website (for registered user; accessed on 20 September 2020): https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

