Thyrotropin N-glycosylation and Glycan Composition in Severe Primary Hypothyroidism
- PMID: 33644618
- PMCID: PMC7896355
- DOI: 10.1210/jendso/bvab006
Thyrotropin N-glycosylation and Glycan Composition in Severe Primary Hypothyroidism
Abstract
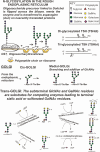
Context: In severe primary hypothyroidism (sPH), the serum thyrotropin (TSH) levels are elevated with an increased degree of sialylation. The circulating TSH comprises 2 different TSH glycoforms: TSHdi with 2 and TSHtri with 3 N-glycans and methods have developed to determine their contents of anionic monosaccharides (AMS), that is, sialic acid (SA) and sulfonated N-acetylglactosamine (SU) residues.
Objective: Characterize N-glycosylation and glycan composition of circulating TSH molecules and determine the effects during levothyroxine treatment in patients with sPH.
Methods: Serum samples were obtained from 25 patients with sPH, from 159 euthyroid individuals, and from 12 women during treatment with levothyroxine for sPH. Degrees of N-glycosylation and concentrations of TSHdi and TSHtri as well as their contents of AMS, SA, and SU residues were determined.
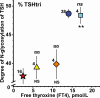
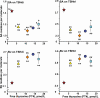
Results: The circulating TSH molecules in sPH patients had lower degrees of N-glycosylation, higher degrees of sialylation, and lower degrees of sulfonation than in euthyroid individuals. Levothyroxin restored sialylation and sulfonation of the glycans already at low free thyroxine (FT4) levels, while degree of N-glycosylation was not restored until the FT4 levels were normal.
Conclusions: The majority of TSH molecules in severe primary hypothyroidism were less N- glycosylated, more sialylated, and less sulfonated compared with euthyroid individuals. This glycan pattern favors a prolonged half-life in the circulation combined with lower in vitro biopotency at the target cells. During levothyroxine treatment of sPH patients, the sialylation and sulfonation of glycans were restored already at low FT4 levels, while N-glycosylation of TSH was not restored until the FT4 levels were normal.
Keywords: N-glycosylation; TSH glycoforms; levothyroxine; primary hypothyroidism; sialic acid; sulfonated N- acetylgalactosamine.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

Similar articles
-
Unique Pattern of N-Glycosylation, Sialylation, and Sulfonation on TSH Molecules in Serum of Children Up to 18 Months.J Clin Endocrinol Metab. 2019 Oct 1;104(10):4651-4659. doi: 10.1210/jc.2018-02576. J Clin Endocrinol Metab. 2019. PMID: 31169903
-
Molecular size and charge as dimensions to identify and characterize circulating glycoforms of human FSH, LH and TSH.Ups J Med Sci. 2017 Nov;122(4):217-223. doi: 10.1080/03009734.2017.1412373. Epub 2018 Jan 4. Ups J Med Sci. 2017. PMID: 29299972 Free PMC article.
-
Modulation of human thyrotropin oligosaccharide structures--enhanced proportion of sialylated and terminally galactosylated serum thyrotropin isoforms in subclinical and overt primary hypothyroidism.J Endocrinol. 1998 Sep;158(3):359-65. doi: 10.1677/joe.0.1580359. J Endocrinol. 1998. PMID: 9846165
-
Diagnosis and treatment of hypothyroidism in TSH deficiency compared to primary thyroid disease: pituitary patients are at risk of under-replacement with levothyroxine.Clin Endocrinol (Oxf). 2011 Jun;74(6):744-9. doi: 10.1111/j.1365-2265.2011.03984.x. Clin Endocrinol (Oxf). 2011. PMID: 21521256
-
Thyroid Function Within the Normal Range, Subclinical Hypothyroidism, and the Risk of Atrial Fibrillation.Circulation. 2017 Nov 28;136(22):2100-2116. doi: 10.1161/CIRCULATIONAHA.117.028753. Epub 2017 Oct 23. Circulation. 2017. PMID: 29061566 Free PMC article. Review.
Cited by
-
Pars Distalis and Pars Tuberalis Thyroid-Stimulating Hormones and Their Roles in Macro-Thyroid-Stimulating Hormone Formation.Int J Mol Sci. 2023 Jul 20;24(14):11699. doi: 10.3390/ijms241411699. Int J Mol Sci. 2023. PMID: 37511458 Free PMC article. Review.
-
Thyroid Stimulating Hormone and Thyroid Hormones (Triiodothyronine and Thyroxine): An American Thyroid Association-Commissioned Review of Current Clinical and Laboratory Status.Thyroid. 2023 Sep;33(9):1013-1028. doi: 10.1089/thy.2023.0169. Epub 2023 Sep 1. Thyroid. 2023. PMID: 37655789 Free PMC article. Review.
References
-
- Miura Y, Perkel VS, Papenberg KA, Johnson MJ, Magner JA. Concanavalin-A, lentil, and ricin lectin affinity binding characteristics of human thyrotropin: differences in the sialylation of thyrotropin in sera of euthyroid, primary, and central hypothyroid patients. J Clin Endocrinol Metab. 1989;69(5):985-995. - PubMed
-
- Papandreou MJ, Persani L, Asteria C, Ronin C, Beck-Peccoz P. Variable carbohydrate structures of circulating thyrotropin as studied by lectin affinity chromatography in different clinical conditions. J Clin Endocrinol Metab. 1993;77(2):393-398. - PubMed
-
- Szkudlinski MW, Thotakura NR, Tropea JE, Grossmann M, Weintraub BD. Asparagine-linked oligosaccharide structures determine clearance and organ distribution of pituitary and recombinant thyrotropin. Endocrinology. 1995;136(8):3325-3330. - PubMed
-
- Trojan J, Theodoropoulou M, Usadel KH, Stalla GK, Schaaf L. Modulation of human thyrotropin oligosaccharide structures–enhanced proportion of sialylated and terminally galactosylated serum thyrotropin isoforms in subclinical and overt primary hypothyroidism. J Endocrinol. 1998;158(3):359-365. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
