Efficacy of indacaterol/glycopyrronium versus salmeterol/fluticasone in current and ex-smokers: a pooled analysis of IGNITE trials
- PMID: 33644225
- PMCID: PMC7897898
- DOI: 10.1183/23120541.00816-2020
Efficacy of indacaterol/glycopyrronium versus salmeterol/fluticasone in current and ex-smokers: a pooled analysis of IGNITE trials
Abstract
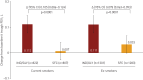
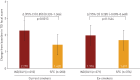
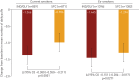
Inhaled corticosteroids have proven to be less effective in asthmatic patients who smoke; however, there is limited information on the efficacy of inhaled corticosteroid-containing regimens in COPD patients who continue smoking. We evaluate the differential efficacy of once-daily indacaterol/glycopyrronium 110/50 µg compared with twice-daily salmeterol/fluticasone 50/500 µg in current smokers and ex-smokers with COPD. A pooled analysis of data from ILLUMINATE, LANTERN and FLAME studies was conducted to assess the efficacy of indacaterol/glycopyrronium compared with salmeterol/fluticasone in current smokers and ex-smokers with COPD. Efficacy was assessed in terms of improvements in trough forced expiratory volume in 1 s (FEV1), transition dyspnoea index (TDI) focal score, St George's Respiratory Questionnaire (SGRQ) total score, reduced rescue medication use and exacerbation prevention at 26 weeks after the start of the therapy. In total, 1769 (38%) current smokers and 2848 (62%) ex-smokers were included. Patients treated with indacaterol/glycopyrronium experienced greater improvements in trough FEV1 versus salmeterol/fluticasone in both current and ex-smokers (least squares mean treatment difference, 105 mL and 78 mL, respectively). Improvements in TDI focal score, SGRQ total score and reduction in rescue medication use were also greater with indacaterol/glycopyrronium versus salmeterol/fluticasone in current and ex-smokers. Furthermore, indacaterol/glycopyrronium reduced all exacerbations (moderate/severe) compared with salmeterol/fluticasone, irrespective of smoking status. The difference in efficacy in favour of indacaterol/glycopyrronium was more prominent in current smokers in most cases. Indacaterol/glycopyrronium demonstrated greater efficacy versus salmeterol/fluticasone, and the differences were generally more prominent in current smokers suggesting smoking may reduce the effects of salmeterol/fluticasone.
Copyright ©ERS 2021.
Conflict of interest statement
Conflict of interest: D.M.G. Halpin reports personal fees from AstraZeneca, personal fees and nonfinancial support from Boehringer Ingelheim, personal fees from Chiesi and GlaxoSmithKline, personal fees and nonfinancial support from Novartis, and personal fees from Pfizer, CSL Behring and Sanofi, outside the submitted work. Conflict of interest: C.F. Vogelmeier reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Grifols, Mundipharma and Novartis, personal fees from Cipla, Berlin Chemie/Menarini, CSL Behring and Teva, and grants from the German Federal Ministry of Education and Research (BMBF) Competence Network Asthma and COPD (ASCONET), Bayer Schering Pharma AG, MSD and Pfizer, outside the submitted work. Conflict of interest: K. Mezzi has nothing to disclose. Conflict of interest: P. Gupta is an employee of Novartis Healthcare Pvt. Ltd. Conflict of interest: K. Kostikas reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, Chiesi, ELPEN, Innovis, Novartis, Menarini and GSK, outside the submitted work; and was an employee of Novartis Pharma AG, Basel during the conduct of this study. Conflict of interest: J.A. Wedzicha reports grants from GSK, and Johnson and Johnson; other support from Novartis, Boehringer Ingelheim, AstraZeneca and GSK; and grants from GSK, AstraZeneca, Boehringer Ingelheim and Novartis, all outside the submitted work.
Figures



Similar articles
-
Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD.N Engl J Med. 2016 Jun 9;374(23):2222-34. doi: 10.1056/NEJMoa1516385. Epub 2016 May 15. N Engl J Med. 2016. PMID: 27181606 Clinical Trial.
-
Efficacy and safety of the direct switch to indacaterol/glycopyrronium from salmeterol/fluticasone in non-frequently exacerbating COPD patients: The FLASH randomized controlled trial.Respirology. 2018 Dec;23(12):1152-1159. doi: 10.1111/resp.13374. Epub 2018 Aug 3. Respirology. 2018. PMID: 30074294 Clinical Trial.
-
Blood Eosinophils and Response to Maintenance Chronic Obstructive Pulmonary Disease Treatment. Data from the FLAME Trial.Am J Respir Crit Care Med. 2017 May 1;195(9):1189-1197. doi: 10.1164/rccm.201701-0193OC. Am J Respir Crit Care Med. 2017. PMID: 28278391 Clinical Trial.
-
Efficacy and safety of a fixed-dose combination of indacaterol and Glycopyrronium for the treatment of COPD: a systematic review.Chest. 2014 Aug;146(2):309-317. doi: 10.1378/chest.13-2807. Chest. 2014. PMID: 24556877 Review.
-
Tiotropium bromide. A review of its use as maintenance therapy in patients with COPD.Treat Respir Med. 2004;3(4):247-68. doi: 10.2165/00151829-200403040-00005. Treat Respir Med. 2004. PMID: 15350163 Review.
Cited by
-
Rational use of inhaled corticosteroids for the treatment of COPD.NPJ Prim Care Respir Med. 2023 Jul 24;33(1):27. doi: 10.1038/s41533-023-00347-6. NPJ Prim Care Respir Med. 2023. PMID: 37488104 Free PMC article. Review.
References
-
- World Health Organization. Chronic respiratory diseases. Causes of COPD www.who.int/respiratory/copd/causes/en/. Date last accessed: January 31, 2021.
LinkOut - more resources
Full Text Sources
