Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production
- PMID: 33639680
- PMCID: PMC8190374
- DOI: 10.31557/APJCP.2021.22.2.603
Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production
Abstract
Background: Low risk of breast cancer is observed among females consuming a moderate quantity of soy throughout their life. The present study was conducted to evaluate the anticancer potential of Daidzein, one of the major Isoflavones in soy using Human breast cancer cells MCF-7.
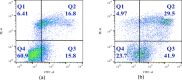
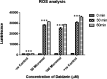
Methods: MCF-7 were subjected to various doses of Daidzein treatment to determine the IC50 value. Onset of apoptosis was ascertained by AnnexinV assay and caspase 3/7 activity post treatment. Expression of pro-apoptotic protein Bax and anti-apoptotic protein Bcl2 was also assessed to further confirm apoptotic mode of cell death. ROS production post treatment with Daidzein was assessed to ascertain the apoptosis via intrinsic pathway. Expression of ER α and ER β was evaluated by western blot analysis.
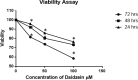
Results: Human breast cancer cells MCF-7 were found to be sensitive to Daidzein treatment, with an IC50 value of 50µM. Increased percentage of treated cells stained with Annexin V confirmed apoptosis mediated cell death. Activity of Caspase 3/7 activity was found to be 1.4-fold higher in Daidzein treated cells than control cells, confirming apoptosis. Daidzein caused over expression of Bax and down-regulated expression of Bcl2. There has been an outburst of ROS in Daidzein treated cells indicating that Daidzein induces apoptosis via intrinsic pathway. A decrease in the expression of ER α and increase in levels of ER β has been observed which are conducive indicator of apoptosis.
Conclusions: In conclusion, the present study suggests that Daidzein induces apoptosis in MCF-7 cells by mitochondrial pathway along with lowering the ratio of ER α/β and an outburst of Reactive Oxygen Species(ROS).
Keywords: Apoptosis; Bax/Bcl-2 Ratio; ER α/β ratio; Molecular docking; ROS.
Figures


Similar articles
-
Daidzein induced apoptosis via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway in BGC-823 cells.Cell Biochem Biophys. 2013 Mar;65(2):197-202. doi: 10.1007/s12013-012-9418-2. Cell Biochem Biophys. 2013. PMID: 22926545
-
Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway.Ann Oncol. 2010 Feb;21(2):263-268. doi: 10.1093/annonc/mdp499. Epub 2009 Nov 4. Ann Oncol. 2010. PMID: 19889614
-
Effects of diverse dietary phytoestrogens on cell growth, cell cycle and apoptosis in estrogen-receptor-positive breast cancer cells.J Nutr Biochem. 2010 Sep;21(9):856-64. doi: 10.1016/j.jnutbio.2009.06.010. Epub 2009 Oct 3. J Nutr Biochem. 2010. PMID: 19800779
-
Mechanisms underlying the dualistic mode of action of major soy isoflavones in relation to cell proliferation and cancer risks.Mol Nutr Food Res. 2013 Jan;57(1):100-13. doi: 10.1002/mnfr.201200439. Epub 2012 Nov 23. Mol Nutr Food Res. 2013. PMID: 23175102 Review.
-
Therapeutic Potential of Isoflavones with an Emphasis on Daidzein.Oxid Med Cell Longev. 2021 Sep 9;2021:6331630. doi: 10.1155/2021/6331630. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34539970 Free PMC article. Review.
Cited by
-
LACTB induces cancer cell death through the activation of the intrinsic caspase-independent pathway in breast cancer.Apoptosis. 2023 Feb;28(1-2):186-198. doi: 10.1007/s10495-022-01775-4. Epub 2022 Oct 25. Apoptosis. 2023. PMID: 36282364 Free PMC article.
-
Phytochemicals Derived from Agricultural Residues and Their Valuable Properties and Applications.Molecules. 2023 Jan 1;28(1):342. doi: 10.3390/molecules28010342. Molecules. 2023. PMID: 36615534 Free PMC article. Review.
-
Gut Microbiota-Assisted Synthesis, Cellular Interactions and Synergistic Perspectives of Equol as a Potent Anticancer Isoflavone.Pharmaceuticals (Basel). 2022 Nov 16;15(11):1418. doi: 10.3390/ph15111418. Pharmaceuticals (Basel). 2022. PMID: 36422548 Free PMC article. Review.
-
Protective Effect of Daidzein against Diethylnitrosamine/Carbon Tetrachloride-Induced Hepatocellular Carcinoma in Male Rats.Biology (Basel). 2023 Aug 29;12(9):1184. doi: 10.3390/biology12091184. Biology (Basel). 2023. PMID: 37759583 Free PMC article.
-
Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms.Int J Mol Sci. 2024 Oct 31;25(21):11713. doi: 10.3390/ijms252111713. Int J Mol Sci. 2024. PMID: 39519265 Free PMC article. Review.
References
-
- Berman HM, Battistuz T, Bhat TN, et al. The protein data bank. Acta Crystallogr D Biol Crystallogr. 2002;58:899–907. - PubMed
-
- Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–42. - PubMed
-
- Choi EJ, Kim G-H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine Int J Phytother Phytopharm. 2008;15:683–90. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

