Thiazole-Based Thiosemicarbazones: Synthesis, Cytotoxicity Evaluation and Molecular Docking Study
- PMID: 33633443
- PMCID: PMC7900779
- DOI: 10.2147/DDDT.S291579
Thiazole-Based Thiosemicarbazones: Synthesis, Cytotoxicity Evaluation and Molecular Docking Study
Abstract
Introduction: Hybrid drug design has developed as a prime method for the development of novel anticancer therapies that can theoretically solve much of the pharmacokinetic disadvantages of traditional anticancer drugs. Thus a number of studies have indicated that thiazole-thiophene hybrids and their bis derivatives have important anticancer activity. Mammalian Rab7b protein is a member of the Rab GTPase protein family that controls the trafficking from endosomes to the TGN. Alteration in the Rab7b expression is implicated in differentiation of malignant cells, causing cancer.
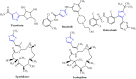
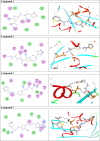
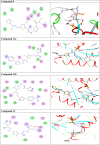
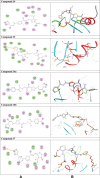
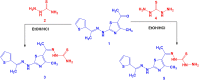
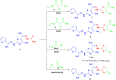
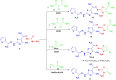
Methods: 1-(4-Methyl-2-(2-(1-(thiophen-2-yl) ethylidene) hydrazinyl) thiazol-5-yl) ethanone was used as building block for synthesis of novel series of 5-(1-(2-(thiazol-2-yl) hydrazono) ethyl) thiazole derivatives. The bioactivities of the synthesized compounds were evaluated with respect to their antitumor activities against MCF-7 tumor cells using MTT assay. Computer-aided docking protocol was performed to study the possible molecular interactions between the newly synthetic thiazole compounds and the active binding site of the target protein Rab7b. Moreover, the in silico prediction of adsorption, distribution, metabolism, excretion (ADME) and toxicity (T) properties of synthesized compounds were carried out using admetSAR tool.
Results: The results obtained showed that derivatives 9 and 11b have promising activity (IC50 = 14.6 ± 0.8 and 28.3 ± 1.5 µM, respectively) compared to Cisplatin (IC50 = 13.6 ± 0.9 µM). The molecular docking analysis reveals that the synthesized compounds are predicted to be fit into the binding site of the target Rab7b. In summary, the synthetic thiazole compounds 1-17 could be used as potent inhibitors as anticancer drugs.
Conclusion: Promising anticancer activity of compounds 9 and 11 compared with cisplatin reference drug suggests that these ligands may contribute as lead compounds in search of new anticancer agents to combat chemo-resistance.
Keywords: MCF-7; Rab7b; docking; hydrazones; hydrazonoyl halides; thiazoles.
© 2021 Gomha et al.
Conflict of interest statement
The authors report no conflicts of interest for this work and declare that there is no conflict of interests regarding the publication of this paper.
Figures

Similar articles
-
Thiazolation of phenylthiosemicarbazone to access new thiazoles: anticancer activity and molecular docking.Future Med Chem. 2024;16(12):1219-1237. doi: 10.1080/17568919.2024.2342668. Epub 2024 May 15. Future Med Chem. 2024. PMID: 38989988
-
Synthesis, Cytotoxicity Evaluation, Molecular Docking and Utility of Novel Chalcones as Precursors for Heterocycles Incorporating Pyrazole Moiety.Med Chem. 2018;14(4):344-355. doi: 10.2174/1573406413666171020114105. Med Chem. 2018. PMID: 29065841
-
Potent ribonucleotide reductase inhibitors: Thiazole-containing thiosemicarbazone derivatives.Arch Pharm (Weinheim). 2019 Nov;352(11):e1900033. doi: 10.1002/ardp.201900033. Epub 2019 Sep 2. Arch Pharm (Weinheim). 2019. PMID: 31475759
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
-
Synthetic and Medicinal Perspective of Fused-Thiazoles as Anticancer Agents.Anticancer Agents Med Chem. 2021;21(11):1379-1402. doi: 10.2174/1871520620666200728133017. Anticancer Agents Med Chem. 2021. PMID: 32723259 Review.
Cited by
-
Synthesis and Molecular Docking of Some Novel 3-Thiazolyl-Coumarins as Inhibitors of VEGFR-2 Kinase.Molecules. 2023 Jan 10;28(2):689. doi: 10.3390/molecules28020689. Molecules. 2023. PMID: 36677750 Free PMC article.
-
Syzygium aromaticum Extracts as a Potential Antibacterial Inhibitors against Clinical Isolates of Acinetobacter baumannii: An In-Silico-Supported In-Vitro Study.Antibiotics (Basel). 2021 Sep 1;10(9):1062. doi: 10.3390/antibiotics10091062. Antibiotics (Basel). 2021. PMID: 34572644 Free PMC article.
-
Optical and Thermal Investigations of New Schiff Base/Ester Systems in Pure and Mixed States.Polymers (Basel). 2021 May 22;13(11):1687. doi: 10.3390/polym13111687. Polymers (Basel). 2021. PMID: 34067245 Free PMC article.
-
New 1,3-diphenyl-1H-pyrazol-5-ols as anti-methicillin resistant Staphylococcus aureus agents: Synthesis, antimicrobial evaluation and in silico studies.Heliyon. 2024 Jun 25;10(13):e33160. doi: 10.1016/j.heliyon.2024.e33160. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39035494 Free PMC article.
-
Synthesis, characterization, in silico molecular docking, and antibacterial activities of some new nitrogen-heterocyclic analogues based on a p-phenolic unit.RSC Adv. 2022 Apr 26;12(20):12607-12621. doi: 10.1039/d2ra01794f. eCollection 2022 Apr 22. RSC Adv. 2022. PMID: 35496342 Free PMC article.
References
-
- Harit T, Bellaouchi R, Asehraou A, Rahal M, Bouabdallah I, Malek F. Synthesis, characterization, antimicrobial activity and theoretical studies of new thiophene-based tripodal ligands. J Mol Struct. 2017;1133:74–79. doi:10.1016/j.molstruc.2016.11.051 - DOI
-
- Mishra P, Middha A, Saxena V, Saxena A. Synthesis and evaluation of anti-inflammatory activity of some cinnoline derivatives-4 (−2-amino-thiophene) cinnoline-3-carboxamide. J Pharm Biosci. 2016;4:64–68. doi:10.20510/ukjpb/4/i3/108388 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

