Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
- PMID: 33631065
- PMCID: PMC7953461
- DOI: 10.1056/NEJMoa2100433
Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19
Abstract
Background: The efficacy of interleukin-6 receptor antagonists in critically ill patients with coronavirus disease 2019 (Covid-19) is unclear.
Methods: We evaluated tocilizumab and sarilumab in an ongoing international, multifactorial, adaptive platform trial. Adult patients with Covid-19, within 24 hours after starting organ support in the intensive care unit (ICU), were randomly assigned to receive tocilizumab (8 mg per kilogram of body weight), sarilumab (400 mg), or standard care (control). The primary outcome was respiratory and cardiovascular organ support-free days, on an ordinal scale combining in-hospital death (assigned a value of -1) and days free of organ support to day 21. The trial uses a Bayesian statistical model with predefined criteria for superiority, efficacy, equivalence, or futility. An odds ratio greater than 1 represented improved survival, more organ support-free days, or both.
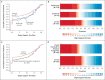
Results: Both tocilizumab and sarilumab met the predefined criteria for efficacy. At that time, 353 patients had been assigned to tocilizumab, 48 to sarilumab, and 402 to control. The median number of organ support-free days was 10 (interquartile range, -1 to 16) in the tocilizumab group, 11 (interquartile range, 0 to 16) in the sarilumab group, and 0 (interquartile range, -1 to 15) in the control group. The median adjusted cumulative odds ratios were 1.64 (95% credible interval, 1.25 to 2.14) for tocilizumab and 1.76 (95% credible interval, 1.17 to 2.91) for sarilumab as compared with control, yielding posterior probabilities of superiority to control of more than 99.9% and of 99.5%, respectively. An analysis of 90-day survival showed improved survival in the pooled interleukin-6 receptor antagonist groups, yielding a hazard ratio for the comparison with the control group of 1.61 (95% credible interval, 1.25 to 2.08) and a posterior probability of superiority of more than 99.9%. All secondary analyses supported efficacy of these interleukin-6 receptor antagonists.
Conclusions: In critically ill patients with Covid-19 receiving organ support in ICUs, treatment with the interleukin-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival. (REMAP-CAP ClinicalTrials.gov number, NCT02735707.).
Copyright © 2021 Massachusetts Medical Society.
Figures

Comment in
-
Interleukin-6 Receptor Inhibition in Covid-19 - Cooling the Inflammatory Soup.N Engl J Med. 2021 Apr 22;384(16):1564-1565. doi: 10.1056/NEJMe2103108. Epub 2021 Feb 25. N Engl J Med. 2021. PMID: 33631064 Free PMC article. No abstract available.
-
Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19.N Engl J Med. 2021 Sep 16;385(12):1147. doi: 10.1056/NEJMc2108482. Epub 2021 Aug 18. N Engl J Med. 2021. PMID: 34407334 No abstract available.
-
COVID-19 and critical care capacity: Can we mitigate demand?Respirology. 2022 Feb;27(2):107-108. doi: 10.1111/resp.14193. Epub 2021 Dec 5. Respirology. 2022. PMID: 34865289 No abstract available.
-
Lessons from the COVID-19 pandemic.Therapie. 2022 Jan-Feb;77(1):3-8. doi: 10.1016/j.therap.2021.12.001. Epub 2021 Dec 4. Therapie. 2022. PMID: 34920865 Free PMC article. No abstract available.
Similar articles
-
Effect of Antiplatelet Therapy on Survival and Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial.JAMA. 2022 Apr 5;327(13):1247-1259. doi: 10.1001/jama.2022.2910. JAMA. 2022. PMID: 35315874 Free PMC article. Clinical Trial.
-
Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients With COVID-19: A Randomized Clinical Trial.JAMA. 2021 Nov 2;326(17):1690-1702. doi: 10.1001/jama.2021.18178. JAMA. 2021. PMID: 34606578 Free PMC article. Clinical Trial.
-
Simvastatin in Critically Ill Patients with Covid-19.N Engl J Med. 2023 Dec 21;389(25):2341-2354. doi: 10.1056/NEJMoa2309995. Epub 2023 Oct 25. N Engl J Med. 2023. PMID: 37888913 Free PMC article. Clinical Trial.
-
Effect of tocilizumab, sarilumab, and baricitinib on mortality among patients hospitalized for COVID-19 treated with corticosteroids: a systematic review and meta-analysis.Clin Microbiol Infect. 2023 Jan;29(1):13-21. doi: 10.1016/j.cmi.2022.07.008. Epub 2022 Jul 19. Clin Microbiol Infect. 2023. PMID: 35863630 Free PMC article. Review.
-
Tocilizumab in COVID-19 pneumonia: Practical proposals based on a narrative review of randomised trials.Rev Med Virol. 2022 Jan;32(1):e2239. doi: 10.1002/rmv.2239. Epub 2021 Apr 21. Rev Med Virol. 2022. PMID: 33882179 Free PMC article. Review.
Cited by
-
Interleukin-1 Beta rs16944 and rs1143634 and Interleukin-6 Receptor rs12083537 Single Nucleotide Polymorphisms as Potential Predictors of COVID-19 Severity.Pathogens. 2024 Oct 21;13(10):915. doi: 10.3390/pathogens13100915. Pathogens. 2024. PMID: 39452786 Free PMC article.
-
Precision Medicine in Acute Respiratory Distress Syndrome: Progress, Challenges, and the Road ahead.Clin Chest Med. 2024 Dec;45(4):835-848. doi: 10.1016/j.ccm.2024.08.005. Epub 2024 Sep 20. Clin Chest Med. 2024. PMID: 39443001 Review.
-
CCL2-mediated endothelial injury drives cardiac dysfunction in long COVID.Nat Cardiovasc Res. 2024 Oct;3(10):1249-1265. doi: 10.1038/s44161-024-00543-8. Epub 2024 Oct 14. Nat Cardiovasc Res. 2024. PMID: 39402206
-
Relationship between SARS-CoV-2 infection and ICU-acquired candidemia in critically ill medical patients: a multicenter prospective cohort study.Crit Care. 2024 Sep 27;28(1):320. doi: 10.1186/s13054-024-05104-w. Crit Care. 2024. PMID: 39334254 Free PMC article.
-
COVID-19 and Cancer Care: A Review and Practical Guide to Caring for Cancer Patients in the Era of COVID-19.Curr Oncol. 2024 Sep 10;31(9):5330-5343. doi: 10.3390/curroncol31090393. Curr Oncol. 2024. PMID: 39330021 Free PMC article. Review.
References
-
- World Health Organization. Coronavirus disease (COVID-19) pandemic. 2021. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 215522/WT_/Wellcome Trust/United Kingdom
- #101003589/Horizon 2020
- RP-2015-06-18/National Institute for Health Research
- #158584/CIHR/Canada
- #602525/Seventh Framework Programme
- RP-2015-06-018/DH_/Department of Health/United Kingdom
- #APP1101719/National Health and Medical Research Council
- K23 GM132688/GM/NIGMS NIH HHS/United States
- PHRC-20-0147/Ministère des Affaires Sociales et de la Santé
- #16/631/Health Research Council of New Zealand
- WT_/Wellcome Trust/United Kingdom
- R35 GM119519/GM/NIGMS NIH HHS/United States
- CTN 2014-012/HRBI_/Health Research Board/Ireland
- CS-2016-16-011/National Institute for Health Research
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
