Enhancement of liver-directed transgene expression at initial and repeat doses of AAV vectors admixed with ImmTOR nanoparticles
- PMID: 33627416
- PMCID: PMC7904260
- DOI: 10.1126/sciadv.abd0321
Enhancement of liver-directed transgene expression at initial and repeat doses of AAV vectors admixed with ImmTOR nanoparticles
Abstract
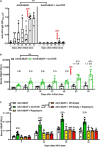
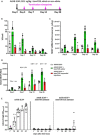
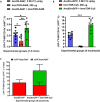
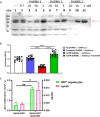
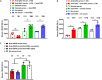
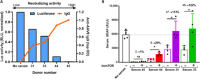
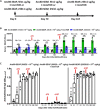
Systemic AAV (adeno-associated virus) gene therapy is a promising approach for the treatment of inborn errors of metabolism, but questions remain regarding its potency and durability. Tolerogenic ImmTOR nanoparticles encapsulating rapamycin have been shown to block the formation of neutralizing anti-capsid antibodies, thereby enabling vector re-administration. Here, we further demonstrate that ImmTOR admixed with AAV vectors also enhances hepatic transgene expression at the initial dose of AAV vector, independent of its effects on adaptive immunity. ImmTOR enhances AAV trafficking to the liver, resulting in increased hepatic vector copy numbers and transgene mRNA expression. Enhanced transgene expression occurs through a mechanism independent of the AAV receptor and cannot be replicated in vivo with free rapamycin or empty nanoparticles. The multipronged mechanism of ImmTOR action makes it an attractive candidate to enable more efficient transgene expression at first dose while simultaneously inhibiting adaptive responses against AAV to enable repeat dosing.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
Similar articles
-
ImmTOR nanoparticles enhance AAV transgene expression after initial and repeat dosing in a mouse model of methylmalonic acidemia.Mol Ther Methods Clin Dev. 2021 Jul 16;22:279-292. doi: 10.1016/j.omtm.2021.06.015. eCollection 2021 Sep 10. Mol Ther Methods Clin Dev. 2021. PMID: 34485611 Free PMC article.
-
Readministration of high-dose adeno-associated virus gene therapy vectors enabled by ImmTOR nanoparticles combined with B cell-targeted agents.PNAS Nexus. 2023 Nov 14;2(11):pgad394. doi: 10.1093/pnasnexus/pgad394. eCollection 2023 Nov. PNAS Nexus. 2023. PMID: 38024395 Free PMC article.
-
Enhancement of the Tolerogenic Phenotype in the Liver by ImmTOR Nanoparticles.Front Immunol. 2021 May 25;12:637469. doi: 10.3389/fimmu.2021.637469. eCollection 2021. Front Immunol. 2021. PMID: 34113339 Free PMC article.
-
Development of ImmTOR Tolerogenic Nanoparticles for the Mitigation of Anti-drug Antibodies.Front Immunol. 2020 May 20;11:969. doi: 10.3389/fimmu.2020.00969. eCollection 2020. Front Immunol. 2020. PMID: 32508839 Free PMC article. Review.
-
Immune responses to adeno-associated virus vectors.Curr Gene Ther. 2005 Jun;5(3):323-31. doi: 10.2174/1566523054065039. Curr Gene Ther. 2005. PMID: 15975009 Review.
Cited by
-
Recombinant Adeno-Associated Virus Vectors for Gene Therapy of the Central Nervous System: Delivery Routes and Clinical Aspects.Biomedicines. 2024 Jul 9;12(7):1523. doi: 10.3390/biomedicines12071523. Biomedicines. 2024. PMID: 39062095 Free PMC article. Review.
-
Synergism of dual AAV gene therapy and rapamycin rescues GSDIII phenotype in muscle and liver.JCI Insight. 2024 May 16;9(11):e172614. doi: 10.1172/jci.insight.172614. JCI Insight. 2024. PMID: 38753465 Free PMC article.
-
Adeno-associated virus as a delivery vector for gene therapy of human diseases.Signal Transduct Target Ther. 2024 Apr 3;9(1):78. doi: 10.1038/s41392-024-01780-w. Signal Transduct Target Ther. 2024. PMID: 38565561 Free PMC article. Review.
-
Role of FoxP3+ Regulatory T Cells in Modulating Immune Responses to Adeno-Associated Virus Gene Therapy.Hum Gene Ther. 2024 Jul;35(13-14):439-450. doi: 10.1089/hum.2023.227. Epub 2024 Apr 11. Hum Gene Ther. 2024. PMID: 38450566 Review.
-
B cell focused transient immune suppression protocol for efficient AAV readministration to the liver.Mol Ther Methods Clin Dev. 2024 Feb 20;32(1):101216. doi: 10.1016/j.omtm.2024.101216. eCollection 2024 Mar 14. Mol Ther Methods Clin Dev. 2024. PMID: 38440160 Free PMC article.
References
-
- Mendell J. R., Al-Zaidy S., Shell R., Arnold W. D., Rodino-Klapac L. R., Prior T. W., Lowes L., Alfano L., Berry K., Church K., Kissel J. T., Nagendran S., L’Italien J., Sproule D. M., Wells C., Cardenas J. A., Heitzer M. D., Kaspar A., Corcoran S., Braun L., Likhite S., Miranda C., Meyer K., Foust K. D., Burghes A. H. M., Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 377, 1713–1722 (2017). - PubMed
-
- Russell S., Bennett J., Wellman J. A., Chung D. C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., Cague S. M., Cross D., Marshall K. A., Walshire J., Kehoe T. L., Reichert H., Davis M., Raffini L., George L. A., Hudson F. P., Dingfield L., Zhu X., Haller J. A., Sohn E. H., Mahajan V. B., Pfeifer W., Weckmann M., Johnson C., Gewaily D., Drack A., Stone E., Wachtel K., Simonelli F., Leroy B. P., Wright J. F., High K. A., Maguire A. M., Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017). - PMC - PubMed
-
- High K. A., Roncarolo M. G., Gene therapy. N. Engl. J. Med. 381, 455–464 (2019). - PubMed
-
- Maguire A. M., Russell S., Wellman J. A., Chung D. C., Yu Z.-F., Tillman A., Wittes J., Pappas J., Elci O., Marshall K. A., Cague S. M., Reichert H., Davis M., Simonelli F., Leroy B. P., Wright J. F., High K. A., Bennett J., Efficacy, safety, and durability of voretigene neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: Results of phase 1 and 3 trials. Ophthalmology 126, 1273–1285 (2019). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

