Modulation of microglial activation states by spinal cord stimulation in an animal model of neuropathic pain: Comparing high rate, low rate, and differential target multiplexed programming
- PMID: 33626981
- PMCID: PMC7925954
- DOI: 10.1177/1744806921999013
Modulation of microglial activation states by spinal cord stimulation in an animal model of neuropathic pain: Comparing high rate, low rate, and differential target multiplexed programming
Abstract
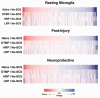
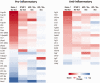
While numerous studies and patient experiences have demonstrated the efficacy of spinal cord stimulation as a treatment for chronic neuropathic pain, the exact mechanism underlying this therapy is still uncertain. Recent studies highlighting the importance of microglial cells in chronic pain and characterizing microglial activation transcriptomes have created a focus on microglia in pain research. Our group has investigated the modulation of gene expression in neurons and glial cells after spinal cord stimulation (SCS), specifically focusing on transcriptomic changes induced by varying SCS stimulation parameters. Previous work showed that, in rodents subjected to the spared nerve injury (SNI) model of neuropathic pain, a differential target multiplexed programming (DTMP) approach provided significantly better relief of pain-like behavior compared to high rate (HRP) and low rate programming (LRP). While these studies demonstrated the importance of transcriptomic changes in SCS mechanism of action, they did not specifically address the role of SCS in microglial activation. The data presented herein utilizes microglia-specific activation transcriptomes to further understand how an SNI model of chronic pain and subsequent continuous SCS treatment with either DTMP, HRP, or LRP affects microglial activation. Genes for each activation transcriptome were identified within our dataset and gene expression levels were compared with that of healthy animals, naïve to injury and interventional procedures. Pearson correlations indicated that DTMP yields the highest significant correlations to expression levels found in the healthy animals across all microglial activation transcriptomes. In contrast, HRP or LRP yielded weak or very weak correlations for these transcriptomes. This work demonstrates that chronic pain and subsequent SCS treatments can modulate microglial activation transcriptomes, supporting previous research on microglia in chronic pain. Furthermore, this study provides evidence that DTMP is more effective than HRP and LRP at modulating microglial transcriptomes, offering potential insight into the therapeutic efficacy of DTMP.
Keywords: chronic neuropathic pain; differential target multiplexed (DTM) programming; microglia transcriptome; spinal cord stimulation.
Conflict of interest statement
Figures
Similar articles
-
Modulation of Glia-Mediated Processes by Spinal Cord Stimulation in Animal Models of Neuropathic Pain.Front Pain Res (Lausanne). 2021 Jul 14;2:702906. doi: 10.3389/fpain.2021.702906. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295479 Free PMC article. Review.
-
Spinal cord stimulation using differential target multiplexed programming modulates neural cell-specific transcriptomes in an animal model of neuropathic pain.Mol Pain. 2020 Jan-Dec;16:1744806920964360. doi: 10.1177/1744806920964360. Mol Pain. 2020. PMID: 33050770 Free PMC article.
-
Effect of stimulation intensity of a differential target multiplexed SCS program in an animal model of neuropathic pain.Pain Pract. 2023 Jul;23(6):639-646. doi: 10.1111/papr.13235. Epub 2023 Apr 17. Pain Pract. 2023. PMID: 37067033
-
Differential target multiplexed spinal cord stimulation programming modulates proteins involved in ion regulation in an animal model of neuropathic pain.Mol Pain. 2022 Jan-Dec;18:17448069211060181. doi: 10.1177/17448069211060181. Mol Pain. 2022. PMID: 35048719 Free PMC article.
-
Spinal Cord Stimulation Paradigms and Pain Relief: A Preclinical Systematic Review on Modulation of the Central Inflammatory Response in Neuropathic Pain.Neuromodulation. 2023 Jan;26(1):25-34. doi: 10.1016/j.neurom.2022.04.049. Epub 2022 Aug 2. Neuromodulation. 2023. PMID: 35931643 Review.
Cited by
-
Answering Big Questions in Pain Medicine.Cureus. 2023 Aug 16;15(8):e43561. doi: 10.7759/cureus.43561. eCollection 2023 Aug. Cureus. 2023. PMID: 37719539 Free PMC article. Review.
-
Modulation of Glia-Mediated Processes by Spinal Cord Stimulation in Animal Models of Neuropathic Pain.Front Pain Res (Lausanne). 2021 Jul 14;2:702906. doi: 10.3389/fpain.2021.702906. eCollection 2021. Front Pain Res (Lausanne). 2021. PMID: 35295479 Free PMC article. Review.
-
Proteomic and Phosphoproteomic Changes of MAPK-Related Inflammatory Response in an Animal Model of Neuropathic Pain by Differential Target Multiplexed SCS and Low-Rate SCS.J Pain Res. 2022 Apr 1;15:895-907. doi: 10.2147/JPR.S348738. eCollection 2022. J Pain Res. 2022. PMID: 35392631 Free PMC article.
-
Regulation of Expression of Extracellular Matrix Proteins by Differential Target Multiplexed Spinal Cord Stimulation (SCS) and Traditional Low-Rate SCS in a Rat Nerve Injury Model.Biology (Basel). 2023 Mar 31;12(4):537. doi: 10.3390/biology12040537. Biology (Basel). 2023. PMID: 37106738 Free PMC article.
-
Differential target multiplexed spinal cord stimulation using a paddle-type lead placed at the appropriate site for neuropathic pain after spinal cord injury in patients with past spinal surgical histories: study protocol for an exploratory clinical trial.Trials. 2023 Jun 13;24(1):395. doi: 10.1186/s13063-023-07433-7. Trials. 2023. PMID: 37308986 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

