Arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression
- PMID: 33626219
- PMCID: PMC8177782
- DOI: 10.1111/cas.14866
Arecoline induces epithelial-mesenchymal transformation and promotes metastasis of oral cancer by SAA1 expression
Abstract
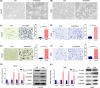
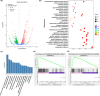
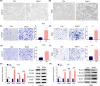
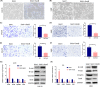
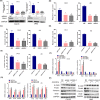
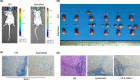
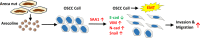
Arecoline, the main alkaloid of areca nut, is well known for its role in inducing submucosal fibrosis and oral squamous cell carcinoma (OSCC), however the mechanism remains unclear. The aim of this study was to establish an arecoline-induced epithelial-mesenchymal transformation (EMT) model of OSCC cells and to investigate the underlying mechanisms. CAL33 and UM2 cells were induced with arecoline to establish an EMT cell model and perform RNA-sequence screening. Luminex multiplex cytokine assays, western blot, and RT-qPCR were used to investigate the EMT mechanism. Arecoline at a concentration of 160 μg/ml was used to induce EMT in OSCC cells, which was confirmed using morphological analysis, transwell assays, and EMT marker detection. RNA-sequence screening and Luminex multiplex cytokine assays showed that many inflammatory cytokines (such as serum amyloid A1 [SAA1], interleukin [IL]-6, IL-36G, chemokine [CCL]2, and CCL20) were significantly altered during arecoline-induced EMT. Of these cytokines, SAA1 was the most highly upregulated. SAA1 overexpression induced EMT and promoted the migration and invasion of CAL33 cells, while SAA1 knockdown attenuated arecoline-induced EMT. Moreover, arecoline enhanced cervical lymph node metastasis in an orthotopic xenograft model of the tongue established using BALB/c nude mice. Our findings revealed that arecoline induced EMT and enhanced the metastatic capability of OSCC by the regulation of inflammatory cytokine secretion, especially that of SAA1. Our study provides a basis for understanding the mechanism of OSCC metastasis and suggests possible therapeutic targets to prevent the occurrence and development of OSCC associated with areca nut chewing.
Keywords: SAA1; arecoline; epithelial-mesenchymal transition; inflammatory cytokines; oral squamous cell carcinoma.
© 2021 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflict of interest.
Figures

Similar articles
-
Acquisition cancer stemness, mesenchymal transdifferentiation, and chemoresistance properties by chronic exposure of oral epithelial cells to arecoline.Oncotarget. 2016 Dec 20;7(51):84072-84081. doi: 10.18632/oncotarget.11432. Oncotarget. 2016. PMID: 27557511 Free PMC article.
-
LncOCMRL1 promotes oral squamous cell carcinoma growth and metastasis via the RRM2/EMT pathway.J Exp Clin Cancer Res. 2024 Sep 30;43(1):267. doi: 10.1186/s13046-024-03190-w. J Exp Clin Cancer Res. 2024. PMID: 39343925 Free PMC article.
-
Fat mass and obesity-associated protein regulates tumorigenesis of arecoline-promoted human oral carcinoma.Cancer Med. 2021 Sep;10(18):6402-6415. doi: 10.1002/cam4.4188. Epub 2021 Aug 11. Cancer Med. 2021. PMID: 34378866 Free PMC article.
-
Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: Challenges and opportunities.Int J Cancer. 2021 Apr 1;148(7):1548-1561. doi: 10.1002/ijc.33352. Epub 2020 Oct 29. Int J Cancer. 2021. PMID: 33091960 Review.
-
Genes involved in the epithelial-mesenchymal transition in oral cancer: A systematic review.Oral Oncol. 2021 Jun;117:105310. doi: 10.1016/j.oraloncology.2021.105310. Epub 2021 Apr 23. Oral Oncol. 2021. PMID: 33901766 Review.
Cited by
-
Transgenic overexpression of the miR-200b/200a/429 cluster inhibits mammary tumor initiation.Transl Oncol. 2021 Dec;14(12):101228. doi: 10.1016/j.tranon.2021.101228. Epub 2021 Sep 22. Transl Oncol. 2021. PMID: 34562686 Free PMC article.
-
Identification of a BRAF/PA28γ/MEK1 signaling axis and its role in epithelial-mesenchymal transition in oral submucous fibrosis.Cell Death Dis. 2022 Aug 12;13(8):701. doi: 10.1038/s41419-022-05152-6. Cell Death Dis. 2022. PMID: 35961969 Free PMC article.
-
Comprehensive insights into areca nut: active components and omics technologies for bioactivity evaluation and quality control.Front Pharmacol. 2024 May 30;15:1407212. doi: 10.3389/fphar.2024.1407212. eCollection 2024. Front Pharmacol. 2024. PMID: 38873426 Free PMC article. Review.
-
A Critical Interpretive Synthesis of the Role of Arecoline in Oral Carcinogenesis: Is the Local Cholinergic Axis a Missing Link in Disease Pathophysiology?Pharmaceuticals (Basel). 2023 Dec 4;16(12):1684. doi: 10.3390/ph16121684. Pharmaceuticals (Basel). 2023. PMID: 38139811 Free PMC article. Review.
-
Deciphering the role of QPCTL in glioma progression and cancer immunotherapy.Front Immunol. 2023 Mar 29;14:1166377. doi: 10.3389/fimmu.2023.1166377. eCollection 2023. Front Immunol. 2023. PMID: 37063864 Free PMC article.
References
-
- Kumar M, Nanavati R, Modi TG, et al. Oral cancer: etiology and risk factors: a review. J Cancer Res Ther. 2016;12(2):458‐463. - PubMed
-
- Chen WQ, Zheng RS, Baade PD, et al. Cancer statistics in China, 2015. CA: A Cancer J Clin, 2016;66(2):115‐132. - PubMed
-
- Siegel RL, Miller KD, Jemal A, et al. Cancer statistics, 2019. CA A Cancer J Clin. 2019;69(1):7‐34. - PubMed
-
- Islam S, Muthumala M, Matsuoka H, et al. How each component of betel quid is involved in oral carcinogenesis: mutual interactions and synergistic effects with other carcinogens–‐a review article. Curr Oncol Rep. 2019;21:53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

