Membrane Rafts: Portals for Viral Entry
- PMID: 33613502
- PMCID: PMC7890030
- DOI: 10.3389/fmicb.2021.631274
Membrane Rafts: Portals for Viral Entry
Abstract
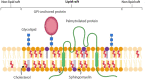
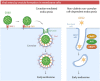
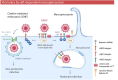
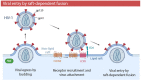
Membrane rafts are dynamic, small (10-200 nm) domains enriched with cholesterol and sphingolipids that compartmentalize cellular processes. Rafts participate in roles essential to the lifecycle of different viral families including virus entry, assembly and/or budding events. Rafts seem to participate in virus attachment and recruitment to the cell surface, as well as the endocytic and non-endocytic mechanisms some viruses use to enter host cells. In this review, we will introduce the specific role of rafts in viral entry and define cellular factors implied in the choice of one entry pathway over the others. Finally, we will summarize the most relevant information about raft participation in the entry process of enveloped and non-enveloped viruses.
Keywords: caveolae; cholesterol; endocytosis; raft; viral entry.
Copyright © 2021 Ripa, Andreu, López-Guerrero and Bello-Morales.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Function of membrane rafts in viral lifecycles and host cellular response.Biochem Res Int. 2011;2011:245090. doi: 10.1155/2011/245090. Epub 2011 Dec 7. Biochem Res Int. 2011. PMID: 22191032 Free PMC article.
-
Cholesterol-rich lipid rafts play a critical role in bovine parainfluenza virus type 3 (BPIV3) infection.Res Vet Sci. 2017 Oct;114:341-347. doi: 10.1016/j.rvsc.2017.04.009. Epub 2017 May 11. Res Vet Sci. 2017. PMID: 28654867
-
Critical role of lipid rafts in virus entry and activation of phosphoinositide 3' kinase/Akt signaling during early stages of Japanese encephalitis virus infection in neural stem/progenitor cells.J Neurochem. 2010 Oct;115(2):537-49. doi: 10.1111/j.1471-4159.2010.06951.x. Epub 2010 Aug 31. J Neurochem. 2010. PMID: 20722967
-
Sphingomyelin and cholesterol: from membrane biophysics and rafts to potential medical applications.Subcell Biochem. 2004;37:167-215. doi: 10.1007/978-1-4757-5806-1_5. Subcell Biochem. 2004. PMID: 15376621 Review.
-
Modulation of entry of enveloped viruses by cholesterol and sphingolipids (Review).Mol Membr Biol. 2003 Jul-Sep;20(3):243-54. doi: 10.1080/0968768031000104944. Mol Membr Biol. 2003. PMID: 12893532 Review.
Cited by
-
Radical-SAM dependent nucleotide dehydratase (SAND), rectification of the names of an ancient iron-sulfur enzyme using NC-IUBMB recommendations.Front Mol Biosci. 2022 Oct 21;9:1032220. doi: 10.3389/fmolb.2022.1032220. eCollection 2022. Front Mol Biosci. 2022. PMID: 36387278 Free PMC article. No abstract available.
-
Antimicrobial Peptides Increase Line Tension in Raft-Forming Lipid Membranes.J Am Chem Soc. 2024 Jul 31;146(30):20891-20903. doi: 10.1021/jacs.4c05377. Epub 2024 Jul 17. J Am Chem Soc. 2024. PMID: 39018511 Free PMC article.
-
Electrostatic Surface Potential as a Key Parameter in Virus Transmission and Evolution: How to Manage Future Virus Pandemics in the Post-COVID-19 Era.Viruses. 2023 Jan 19;15(2):284. doi: 10.3390/v15020284. Viruses. 2023. PMID: 36851498 Free PMC article. Review.
-
Regulation of cholesterol homeostasis in health and diseases: from mechanisms to targeted therapeutics.Signal Transduct Target Ther. 2022 Aug 2;7(1):265. doi: 10.1038/s41392-022-01125-5. Signal Transduct Target Ther. 2022. PMID: 35918332 Free PMC article. Review.
-
Biosynthesis, structure and biological function of cholesterol glucoside in Helicobacter pylori: A review.Medicine (Baltimore). 2023 Sep 8;102(36):e34911. doi: 10.1097/MD.0000000000034911. Medicine (Baltimore). 2023. PMID: 37682174 Free PMC article. Review.
References
-
- Anderson H. A., Chen Y., Norkin L. C. (1998). MHC class I molecules are enriched in caveolae but do not enter with simian virus 40. J. Gen. Virol. 79 1469–1477. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

