Development of a SARS-CoV-2-derived receptor-binding domain-based ACE2 biosensor
- PMID: 33612970
- PMCID: PMC7885701
- DOI: 10.1016/j.snb.2021.129663
Development of a SARS-CoV-2-derived receptor-binding domain-based ACE2 biosensor
Abstract
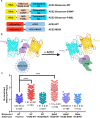
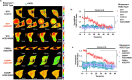
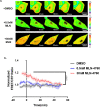
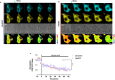
The global outbreak of coronavirus disease and rapid spread of the causative severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represent a significant threat to human health. A key mechanism of human SARS-CoV-2 infection is initiated by the combination of human angiotensin-converting enzyme 2 (hACE2) and the receptor-binding domain (RBD) of the SARS-CoV-2-derived spike glycoprotein. Despite the importance of these protein interactions, there are still insufficient detection methods to observe their activity at the cellular level. Herein, we developed a novel fluorescence resonance energy transfer (FRET)-based hACE2 biosensor to monitor the interaction between hACE2 and SARS-CoV-2 RBD. This biosensor facilitated the visualization of hACE2-RBD activity with high spatiotemporal resolutions at the single-cell level. Further studies revealed that the FRET-based hACE2 biosensors were sensitive to both exogenous and endogenous hACE2 expression, suggesting that they might be safely applied to the early stage of SARS-CoV-2 infection without direct virus use. Therefore, our novel biosensor could potentially help develop drugs that target SARS-CoV-2 by inhibiting hACE2-RBD interaction.
Keywords: ACE2; Biosensor; CQ, chloroquine; FRET; HCQ, hydroxychloroquine; Live-cell imaging; NA, numerical aperture; RBD, receptor-binding domain; RBM, receptor-binding motif; ROI, region of interest; SARS-CoV-2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEM, standard error of the mean; bg, background; hACE2, human angiotensin-converting enzyme 2.
© 2021 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Role of tannic acid against SARS-cov-2 cell entry by targeting the interface region between S-protein-RBD and human ACE2.Front Pharmacol. 2022 Aug 8;13:940628. doi: 10.3389/fphar.2022.940628. eCollection 2022. Front Pharmacol. 2022. PMID: 36003511 Free PMC article.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
-
FRET-based hACE2 receptor mimic peptide conjugated nanoprobe for simple detection of SARS-CoV-2.Chem Eng J. 2022 Aug 15;442:136143. doi: 10.1016/j.cej.2022.136143. Epub 2022 Mar 31. Chem Eng J. 2022. PMID: 35382003 Free PMC article.
-
A novel screening strategy of anti-SARS-CoV-2 drugs via blocking interaction between Spike RBD and ACE2.Environ Int. 2021 Feb;147:106361. doi: 10.1016/j.envint.2020.106361. Epub 2020 Dec 23. Environ Int. 2021. PMID: 33401173 Free PMC article.
-
Interactions of angiotensin-converting enzyme-2 (ACE2) and SARS-CoV-2 spike receptor-binding domain (RBD): a structural perspective.Mol Biol Rep. 2023 Mar;50(3):2713-2721. doi: 10.1007/s11033-022-08193-4. Epub 2022 Dec 23. Mol Biol Rep. 2023. PMID: 36562937 Free PMC article. Review.
Cited by
-
Screening of potential spike glycoprotein / ACE2 dual antagonists against COVID-19 in silico molecular docking.J Virol Methods. 2022 Mar;301:114424. doi: 10.1016/j.jviromet.2021.114424. Epub 2021 Dec 10. J Virol Methods. 2022. PMID: 34896453 Free PMC article.
-
Angiotensin-Converting Enzyme 2-Based Biosensing Modalities and Devices for Coronavirus Detection.Biosensors (Basel). 2022 Nov 7;12(11):984. doi: 10.3390/bios12110984. Biosensors (Basel). 2022. PMID: 36354493 Free PMC article. Review.
-
A Biosensor Platform for Point-of-Care SARS-CoV-2 Screening.Biosensors (Basel). 2022 Jul 3;12(7):487. doi: 10.3390/bios12070487. Biosensors (Basel). 2022. PMID: 35884290 Free PMC article.
-
Low-Cost Biosensor Technologies for Rapid Detection of COVID-19 and Future Pandemics.ACS Nano. 2024 Jan 23;18(3):1757-1777. doi: 10.1021/acsnano.3c01629. Epub 2024 Jan 8. ACS Nano. 2024. PMID: 38189684 Free PMC article. Review.
-
Development of Fluorescence-Based Assays for Key Viral Proteins in the SARS-CoV-2 Infection Process and Lifecycle.Int J Mol Sci. 2024 Mar 1;25(5):2850. doi: 10.3390/ijms25052850. Int J Mol Sci. 2024. PMID: 38474097 Free PMC article. Review.
References
-
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001316. - DOI - PMC - PubMed
-
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. - DOI - PMC - PubMed
-
- Xu X., Yu C., Qu J., Zhang L., Jiang S., Huang D., Chen B., Zhang Z., Guan W., Ling Z., Jiang R., Hu T., Ding Y., Lin L., Gan Q., Luo L., Tang X., Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur. J. Nucl. Med. Mol. Imaging. 2020 doi: 10.1007/s00259-020-04735-9. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

