Autophagy inhibitors increase the susceptibility of KRAS-mutant human colorectal cancer cells to a combined treatment of 2-deoxy-D-glucose and lovastatin
- PMID: 33608672
- PMCID: PMC8564510
- DOI: 10.1038/s41401-021-00612-9
Autophagy inhibitors increase the susceptibility of KRAS-mutant human colorectal cancer cells to a combined treatment of 2-deoxy-D-glucose and lovastatin
Erratum in
-
Author Correction: Autophagy inhibitors increase the susceptibility of KRAS-mutant human colorectal cancer cells to a combined treatment of 2-deoxy-D-glucose and lovastatin.Acta Pharmacol Sin. 2022 Jul;43(7):1881. doi: 10.1038/s41401-021-00834-x. Acta Pharmacol Sin. 2022. PMID: 34873318 Free PMC article. No abstract available.
Abstract
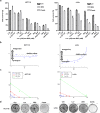
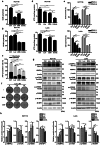
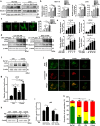
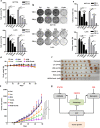
RAS-driven colorectal cancer relies on glucose metabolism to support uncontrolled growth. However, monotherapy with glycolysis inhibitors like 2-deoxy-D-glucose causes limited effectiveness. Recent studies suggest that anti-tumor effects of glycolysis inhibition could be improved by combination treatment with inhibitors of oxidative phosphorylation. In this study we investigated the effect of a combination of 2-deoxy-D-glucose with lovastatin (a known inhibitor of mevalonate pathway and oxidative phosphorylation) on growth of KRAS-mutant human colorectal cancer cell lines HCT116 and LoVo. A combination of lovastatin (>3.75 μM) and 2-deoxy-D-glucose (>1.25 mM) synergistically reduced cell viability, arrested cells in the G2/M phase, and induced apoptosis. The combined treatment also reduced cellular oxygen consumption and extracellular acidification rate, resulting in decreased production of ATP and lower steady-state ATP levels. Energy depletion markedly activated AMPK, inhibited mTOR and RAS signaling pathways, eventually inducing autophagy, the cellular pro-survival process under metabolic stress, whereas inhibition of autophagy by chloroquine (6.25 μM) enhanced the cytotoxic effect of the combination of lovastatin and 2-deoxy-D-glucose. These in vitro experiment results were reproduced in a nude mouse xenograft model of HCT116 cells. Our findings suggest that concurrently targeting glycolysis, oxidative phosphorylation, and autophagy may be a promising regimen for the management of RAS-driven colorectal cancers.
Keywords: 2DG; OXPHOS; autophagy; chloroquine; glycolysis; human colorectal cancers; hydroxychloroquine; lovastatin.
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Dasatinib sensitizes KRAS mutant colorectal tumors to cetuximab.Oncogene. 2011 Feb 3;30(5):561-74. doi: 10.1038/onc.2010.430. Epub 2010 Oct 18. Oncogene. 2011. PMID: 20956938 Free PMC article.
-
PCC0208023, a potent SHP2 allosteric inhibitor, imparts an antitumor effect against KRAS mutant colorectal cancer.Toxicol Appl Pharmacol. 2020 Jul 1;398:115019. doi: 10.1016/j.taap.2020.115019. Epub 2020 Apr 24. Toxicol Appl Pharmacol. 2020. PMID: 32335126
-
Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation.Theranostics. 2020 Apr 6;10(11):5107-5119. doi: 10.7150/thno.44705. eCollection 2020. Theranostics. 2020. PMID: 32308771 Free PMC article.
-
Fangchinoline exerts anticancer effects on colorectal cancer by inducing autophagy via regulation AMPK/mTOR/ULK1 pathway.Biochem Pharmacol. 2021 Apr;186:114475. doi: 10.1016/j.bcp.2021.114475. Epub 2021 Feb 18. Biochem Pharmacol. 2021. PMID: 33609560
-
Unraveling and targeting RAS-driven metabolic signaling for therapeutic gain.Adv Cancer Res. 2022;153:267-304. doi: 10.1016/bs.acr.2021.07.010. Epub 2021 Aug 26. Adv Cancer Res. 2022. PMID: 35101233 Review.
Cited by
-
Mitochondrial toxicity evaluation of traditional Chinese medicine injections with a dual in vitro approach.Front Pharmacol. 2022 Nov 2;13:1039235. doi: 10.3389/fphar.2022.1039235. eCollection 2022. Front Pharmacol. 2022. PMID: 36408232 Free PMC article.
-
Yinzhihuang injection induces apoptosis and suppresses tumor growth in acute myeloid leukemia cells.PLoS One. 2023 Oct 10;18(10):e0289697. doi: 10.1371/journal.pone.0289697. eCollection 2023. PLoS One. 2023. PMID: 37816017 Free PMC article.
-
Crucial Role of Oncogenic KRAS Mutations in Apoptosis and Autophagy Regulation: Therapeutic Implications.Cells. 2022 Jul 13;11(14):2183. doi: 10.3390/cells11142183. Cells. 2022. PMID: 35883626 Free PMC article. Review.
-
Identification of multiple risk factors for colorectal cancer relapse after laparoscopic radical resection.World J Gastrointest Surg. 2023 Oct 27;15(10):2211-2221. doi: 10.4240/wjgs.v15.i10.2211. World J Gastrointest Surg. 2023. PMID: 37969700 Free PMC article.
-
Recent Developments in Targeting RAS Downstream Effectors for RAS-Driven Cancer Therapy.Molecules. 2021 Dec 14;26(24):7561. doi: 10.3390/molecules26247561. Molecules. 2021. PMID: 34946644 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

