Mechanisms for Regulating and Organizing Receptor Signaling by Endocytosis
- PMID: 33606955
- PMCID: PMC8608402
- DOI: 10.1146/annurev-biochem-081820-092427
Mechanisms for Regulating and Organizing Receptor Signaling by Endocytosis
Abstract
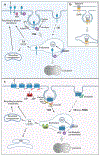
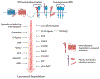
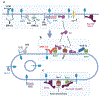
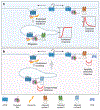
Intricate relationships between endocytosis and cellular signaling, first recognized nearly 40 years ago through the study of tyrosine kinase growth factor receptors, are now known to exist for multiple receptor classes and to affect myriad physiological and developmental processes. This review summarizes our present understanding of how endocytosis orchestrates cellular signaling networks, with an emphasis on mechanistic underpinnings and focusing on two receptor classes-tyrosine kinase and G protein-coupled receptors-that have been investigated in particular detail. Together, these examples provide a useful survey of the current consensus, uncertainties, and controversies in this rapidly advancing area of cell biology.
Keywords: G protein–coupled receptors; downregulation; endocytosis; endosomes; receptor tyrosine kinases; signaling.
Figures
Similar articles
-
Intracellular trafficking of hormone receptors.Trends Endocrinol Metab. 2004 Aug;15(6):286-93. doi: 10.1016/j.tem.2004.06.009. Trends Endocrinol Metab. 2004. PMID: 15358282 Review.
-
Endocytosis of receptor tyrosine kinases.Cold Spring Harb Perspect Biol. 2013 May 1;5(5):a017459. doi: 10.1101/cshperspect.a017459. Cold Spring Harb Perspect Biol. 2013. PMID: 23637288 Free PMC article. Review.
-
Receptor tyrosine kinases endocytosis in endothelium: biology and signaling.Arterioscler Thromb Vasc Biol. 2014 Sep;34(9):1831-7. doi: 10.1161/ATVBAHA.114.303217. Epub 2014 Jun 12. Arterioscler Thromb Vasc Biol. 2014. PMID: 24925972 Free PMC article. Review.
-
Regulation of receptor tyrosine kinase signaling by endocytic trafficking.Traffic. 2001 Jan;2(1):12-8. doi: 10.1034/j.1600-0854.2001.020103.x. Traffic. 2001. PMID: 11208164 Review.
-
Receptor tyrosine kinase and G-protein coupled receptor signaling and sorting within endosomes.J Neurochem. 2003 Mar;84(5):905-18. doi: 10.1046/j.1471-4159.2003.01603.x. J Neurochem. 2003. PMID: 12603816 Review. No abstract available.
Cited by
-
Mechanism of p38 MAPK-induced EGFR endocytosis and its crosstalk with ligand-induced pathways.J Cell Biol. 2021 Jul 5;220(7):e202102005. doi: 10.1083/jcb.202102005. Epub 2021 May 25. J Cell Biol. 2021. PMID: 34032851 Free PMC article.
-
CRISPR/Cas9 Gene Editing of HeLa Cells to Tag Proteins with mNeonGreen.Bio Protoc. 2022 May 20;12(10):e4415. doi: 10.21769/BioProtoc.4415. eCollection 2022 May 20. Bio Protoc. 2022. PMID: 35813028 Free PMC article.
-
Distinct beta-arrestin coupling and intracellular trafficking of metabotropic glutamate receptor homo- and heterodimers.Sci Adv. 2023 Dec 8;9(49):eadi8076. doi: 10.1126/sciadv.adi8076. Epub 2023 Dec 6. Sci Adv. 2023. PMID: 38055809 Free PMC article.
-
The role of endocytic trafficking in antigen T cell receptor activation.Biomed J. 2022 Apr;45(2):310-320. doi: 10.1016/j.bj.2021.09.004. Epub 2021 Sep 28. Biomed J. 2022. PMID: 34592497 Free PMC article. Review.
-
Loss of tumor suppressor TMEM127 drives RET-mediated transformation through disrupted membrane dynamics.Elife. 2024 Apr 30;12:RP89100. doi: 10.7554/eLife.89100. Elife. 2024. PMID: 38687678 Free PMC article.
References
-
- Bergeron JJM, Di Guglielmo GM, Dahan S, Dominguez M, Posner BI. 2016. Spatial and temporal regulation of receptor tyrosine kinase activation and intracellular signal transduction. Annu. Rev. Biochem. 85:573–97 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

