Certolizumab Pegol for the Treatment of Moderate to Severe Plaque Psoriasis: 16-Week Results from a Phase 2/3 Japanese Study
- PMID: 33606269
- PMCID: PMC8019007
- DOI: 10.1007/s13555-021-00494-z
Certolizumab Pegol for the Treatment of Moderate to Severe Plaque Psoriasis: 16-Week Results from a Phase 2/3 Japanese Study
Abstract
Introduction: Certolizumab pegol (CZP), the Fc-free, PEGylated anti-tumor necrosis factor, is approved for the treatment of moderate to severe plaque psoriasis (PSO) in Western countries and in Japan, among other indications.
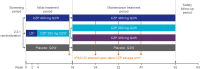
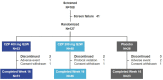
Methods: We report results from the first 16 weeks of a 52-week phase 2/3 trial of CZP in Japanese patients with PSO. Patients ≥ 20 years with PSO ≥ 6 months (Psoriasis Area and Severity Index [PASI] ≥ 12, body surface area affected ≥ 10%, and Physician's Global Assessment [PGA] ≥ 3 on a 5-point scale) were randomized 2:2:1 to CZP 400 mg every 2 weeks (Q2W), CZP 200 mg Q2W (400 mg weeks 0/2/4), or placebo Q2W. Outcomes assessed to week 16: PASI 75, PASI 90, PGA 0/1 (Markov chain Monte Carlo), Dermatology Life Quality Index (DLQI 0/1) and Itch Numeric Rating Scale (INRS 0) (non-responder imputation), and DLQI and INRS change from baseline (last observation carried forward). Safety data were reported for patients receiving ≥ 1 dose of study medication through weeks 0-16; adverse events were evaluated using Medical Dictionary for Regulatory Activities version 18.1.
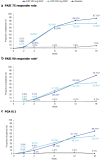
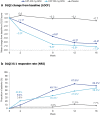
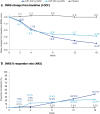
Results: A total of 127 patients were randomized to CZP 400 mg Q2W (N = 53), CZP 200 mg Q2W (N = 48), placebo (N = 26). Week 16 responder rates for CZP 400 mg/200 mg Q2W versus placebo were 87.1%/73.0% versus 7.9% for PASI 75; 75.7%/53.8% versus 0.2% for PASI 90; 66.7%/52.7% versus 0.0% for PGA 0/1 (all p < 0.0001 for both CZP doses versus placebo). Significant improvements in DLQI and INRS were reported at week 16 by patients receiving both CZP doses compared with placebo (p < 0.0001). Incidence of treatment-emergent adverse events within the CZP 400 mg Q2W, CZP 200 mg Q2W, and placebo groups were 326.1, 404.9, and 682.4 per 100 patient-years. No new safety signals were identified compared to previously reported data.
Conclusion: CZP dosed at 400 mg or 200 mg Q2W was associated with improved PSO signs and symptoms.
Trial registration: ClinicalTrials.gov identifier, NCT03051217.
Keywords: Certolizumab pegol; Japan; Plaque psoriasis; Tumor necrosis factor-alpha.
Figures
Similar articles
-
Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Generalized Pustular Psoriasis and Erythrodermic Psoriasis: 52-Week Results.Dermatol Ther (Heidelb). 2022 Jun;12(6):1397-1415. doi: 10.1007/s13555-022-00741-x. Epub 2022 May 27. Dermatol Ther (Heidelb). 2022. PMID: 35622315 Free PMC article.
-
Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Moderate to Severe Plaque Psoriasis: 52-Week Results.Dermatol Ther (Heidelb). 2021 Jun;11(3):943-960. doi: 10.1007/s13555-021-00520-0. Epub 2021 Apr 22. Dermatol Ther (Heidelb). 2021. PMID: 33886085 Free PMC article.
-
Certolizumab Pegol in Japanese Patients with Moderate to Severe Plaque Psoriasis: Effect of Demographics and Baseline Disease Characteristics on Efficacy.Dermatol Ther (Heidelb). 2022 Jan;12(1):121-135. doi: 10.1007/s13555-021-00645-2. Epub 2021 Nov 26. Dermatol Ther (Heidelb). 2022. PMID: 34826124 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated. Review.
-
Certolizumab Pegol: A Review in Moderate to Severe Plaque Psoriasis.BioDrugs. 2020 Apr;34(2):235-244. doi: 10.1007/s40259-020-00416-z. BioDrugs. 2020. PMID: 32207094 Review.
Cited by
-
Current Treatments for Generalized Pustular Psoriasis: A Narrative Summary of a Systematic Literature Search.Dermatol Ther (Heidelb). 2024 Sep;14(9):2331-2378. doi: 10.1007/s13555-024-01230-z. Epub 2024 Aug 1. Dermatol Ther (Heidelb). 2024. PMID: 39088126 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2023 Jul 12;7(7):CD011535. doi: 10.1002/14651858.CD011535.pub6. Cochrane Database Syst Rev. 2023. PMID: 37436070 Free PMC article. Review.
-
Short-, Mid-, and Long-Term Efficacy of Deucravacitinib Versus Biologics and Nonbiologics for Plaque Psoriasis: A Network Meta-Analysis.Dermatol Ther (Heidelb). 2023 Nov;13(11):2839-2857. doi: 10.1007/s13555-023-01034-7. Epub 2023 Oct 6. Dermatol Ther (Heidelb). 2023. PMID: 37801281 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2022 May 23;5(5):CD011535. doi: 10.1002/14651858.CD011535.pub5. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2023 Jul 12;7:CD011535. doi: 10.1002/14651858.CD011535.pub6. PMID: 35603936 Free PMC article. Updated. Review.
-
Efficacy and Safety of Certolizumab Pegol in Japanese Patients with Generalized Pustular Psoriasis and Erythrodermic Psoriasis: 52-Week Results.Dermatol Ther (Heidelb). 2022 Jun;12(6):1397-1415. doi: 10.1007/s13555-022-00741-x. Epub 2022 May 27. Dermatol Ther (Heidelb). 2022. PMID: 35622315 Free PMC article.
References
-
- National Psoriasis Foundation: Plaque Psoriasis. 2019. https://www.psoriasis.org/about-psoriasis/types/plaque. Accessed 4 Sept 2019.
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

