CBP Bromodomain Inhibition Rescues Mice From Lethal Sepsis Through Blocking HMGB1-Mediated Inflammatory Responses
- PMID: 33603756
- PMCID: PMC7884462
- DOI: 10.3389/fimmu.2020.625542
CBP Bromodomain Inhibition Rescues Mice From Lethal Sepsis Through Blocking HMGB1-Mediated Inflammatory Responses
Abstract
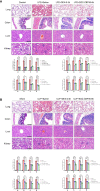
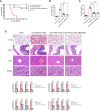
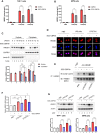
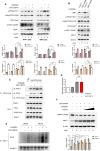
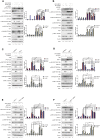
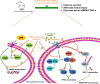
CREB binding protein (CBP), a transcriptional coactivator and acetyltransferase, is involved in the pathogenesis of inflammation-related diseases. High mobility group box-1 protein (HMGB1) is a critical mediator of lethal sepsis, which has prompted investigation for the development of new treatment for inflammation. Here, we report that the potent and selective inhibition of CBP bromodomain by SGC-CBP30 blocks HMGB1-mediated inflammatory responses in vitro and in vivo. Our data suggest that CBP bromodomain inhibition suppresses LPS-induced expression and release of HMGB1, when the inhibitor was given 8 h post LPS stimulation; moreover, CBP bromodomain inhibition attenuated pro-inflammatory activity of HMGB1. Furthermore, our findings provide evidence that SGC-CBP30 down-regulated rhHMGB1-induced activation of MAPKs and NF-κB signaling by triggering the reactivation of protein phosphatase 2A (PP2A) and the stabilization of MAPK phosphatase 1 (MKP-1). Collectively, these results suggest that CBP bromodomain could serve as a candidate therapeutic target for the treatment of lethal sepsis via inhibiting LPS-induced expression and release of HMGB1 and suppressing the pro-inflammatory activity of HMGB1.
Keywords: CREB binding protein; MAPK phosphatase 1; high mobility group box-1 protein; protein phosphatase 2A; sepsis.
Copyright © 2021 Bi, Jiang, Zhou, Fan, Yan, Liang, Luo and Yin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Indoprofen exerts a potent therapeutic effect against sepsis by alleviating high mobility group box 1-mediated inflammatory responses.Toxicol Appl Pharmacol. 2021 Dec 15;433:115778. doi: 10.1016/j.taap.2021.115778. Epub 2021 Oct 29. Toxicol Appl Pharmacol. 2021. PMID: 34755645
-
Ameliorative effect of a rarely occurring C-methylrotenoid on HMGB1-induced septic responses in vitro and in vivo.Biochem Pharmacol. 2016 Jun 15;110-111:58-70. doi: 10.1016/j.bcp.2016.04.006. Epub 2016 Apr 19. Biochem Pharmacol. 2016. PMID: 27106082
-
Quercetin prevents LPS-induced high-mobility group box 1 release and proinflammatory function.Am J Respir Cell Mol Biol. 2009 Dec;41(6):651-60. doi: 10.1165/rcmb.2008-0119OC. Epub 2009 Mar 5. Am J Respir Cell Mol Biol. 2009. PMID: 19265175 Free PMC article.
-
The Regulatory Role of High-Mobility Group Protein 1 in Sepsis-Related Immunity.Front Immunol. 2021 Jan 22;11:601815. doi: 10.3389/fimmu.2020.601815. eCollection 2020. Front Immunol. 2021. PMID: 33552058 Free PMC article. Review.
-
The cytokine activity of HMGB1.J Leukoc Biol. 2005 Jul;78(1):1-8. doi: 10.1189/jlb.1104648. Epub 2005 Feb 25. J Leukoc Biol. 2005. PMID: 15734795 Review.
Cited by
-
Cryptotanshinone attenuates LPS-induced acute lung injury by regulating metabolic reprogramming of macrophage.Front Med (Lausanne). 2023 Jan 13;9:1075465. doi: 10.3389/fmed.2022.1075465. eCollection 2022. Front Med (Lausanne). 2023. PMID: 36714100 Free PMC article.
-
Single-cell transcriptomics reveals intestinal cell heterogeneity and identifies Ep300 as a potential therapeutic target in mice with acute liver failure.Cell Discov. 2023 Jul 25;9(1):77. doi: 10.1038/s41421-023-00578-4. Cell Discov. 2023. PMID: 37488127 Free PMC article.
-
The β-catenin/CBP signaling axis participates in sepsis-induced inflammatory lung injury.Exp Biol Med (Maywood). 2022 Sep;247(17):1548-1557. doi: 10.1177/15353702221097316. Epub 2022 Jun 6. Exp Biol Med (Maywood). 2022. PMID: 35665630 Free PMC article.
-
P4HA3 promotes colon cancer cell escape from macrophage phagocytosis by increasing phagocytosis immune checkpoint CD47 expression.Mol Cell Biochem. 2024 Dec;479(12):3355-3374. doi: 10.1007/s11010-024-04927-z. Epub 2024 Feb 12. Mol Cell Biochem. 2024. PMID: 38347264
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

