miR-29b restrains cholangiocarcinoma progression by relieving DNMT3B-mediated repression of CDKN2B expression
- PMID: 33601338
- PMCID: PMC7950249
- DOI: 10.18632/aging.202549
miR-29b restrains cholangiocarcinoma progression by relieving DNMT3B-mediated repression of CDKN2B expression
Abstract
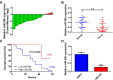
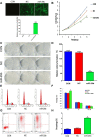
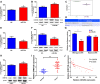
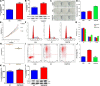
Numerous studies have reported the important role of microRNAs (miRNAs) in human cancers. Although abnormal miR-29b expression has been linked to tumorigenesis in several cancers, its role in cholangiocarcinoma remains largely unknown. We found that miR-29b expression is frequently downregulated in human cholangiocarcinoma QBC939 cells and in clinical tumor samples. In cholangiocarcinoma patients, low miR-29b expression predicts poor overall survival. Overexpression of miR-29b in QBC939 cells inhibited proliferation, induced G1 phase cycle arrest, and promoted apoptosis. Methylation-specific PCR (MSP) analysis revealed a decreased methylation imprint at the promoter of the cell cycle inhibitor gene CDKN2B in cells overexpressing miR-29b. After identifying the DNA methyltransferase DNMT3B as a putative miR-29b target, luciferase reporter assays confirmed a suppressive effect of miR-29b on DNMT3B expression. Accordingly, we detected an inverse correlation between miR-29b and DNMT3B expression in clinical cholangiocarcinoma specimens. In QBC939 cells, DNMT3B overexpression promoted proliferation and inhibited apoptosis. DNMT3B silencing, in turn, led to increased CDKN2B expression. We also observed significant growth arrest in subcutaneous tumors formed in nude mice by QBC939 cells overexpressing miR-29b. These findings suggest miR-29b functions as a tumor suppressor in cholangiocarcinoma by relieving DNMT3B-mediated repression of CDKN2B expression.
Keywords: CDKN2B; DNMT3B; cholangiocarcinoma; methylation; miR-29b.
Conflict of interest statement
Figures
Similar articles
-
Regulation of DNA methylation and tumor suppression gene expression by miR-29b in leukemia patients and related mechanisms.Eur Rev Med Pharmacol Sci. 2018 Jan;22(1):158-165. doi: 10.26355/eurrev_201801_14113. Eur Rev Med Pharmacol Sci. 2018. PMID: 29364483
-
[Overpression of miR-29b suppresses the proliferation and induces apoptosis of cholangiocarcinoma cells].Nan Fang Yi Ke Da Xue Xue Bao. 2018 Sep 30;38(10):1234-1238. doi: 10.3969/j.issn.1673-4254.2018.10.13. Nan Fang Yi Ke Da Xue Xue Bao. 2018. PMID: 30377134 Free PMC article. Chinese.
-
Downregulation of microRNA-29b by DNMT3B decelerates chondrocyte apoptosis and the progression of osteoarthritis via PTHLH/CDK4/RUNX2 axis.Aging (Albany NY). 2020 Nov 7;13(5):7676-7690. doi: 10.18632/aging.103778. Epub 2020 Nov 7. Aging (Albany NY). 2020. PMID: 33177241 Free PMC article.
-
MicroRNA-29b induces global DNA hypomethylation and tumor suppressor gene reexpression in acute myeloid leukemia by targeting directly DNMT3A and 3B and indirectly DNMT1.Blood. 2009 Jun 18;113(25):6411-8. doi: 10.1182/blood-2008-07-170589. Epub 2009 Feb 11. Blood. 2009. PMID: 19211935 Free PMC article.
-
mir-637 inhibits the proliferation of cholangiocarcinoma cell QBC939 through interfering CTSB expression.Eur Rev Med Pharmacol Sci. 2018 Mar;22(5):1265-1276. doi: 10.26355/eurrev_201803_14467. Eur Rev Med Pharmacol Sci. 2018. PMID: 29565483
Cited by
-
Non-coding RNAs/DNMT3B axis in human cancers: from pathogenesis to clinical significance.J Transl Med. 2023 Sep 13;21(1):621. doi: 10.1186/s12967-023-04510-y. J Transl Med. 2023. PMID: 37705098 Free PMC article. Review.
-
Role of gga-miR-29b-3p in suppressing the proliferation, invasion and migration of MSB1 Marek's disease tumor cells by the targeting of the DNMT3B gene.Ann Transl Med. 2022 Aug;10(16):873. doi: 10.21037/atm-22-3519. Ann Transl Med. 2022. PMID: 36110994 Free PMC article.
-
Low-level gastrokine 2 promoted progress of NSCLC and as a potential biomarker.J Clin Lab Anal. 2022 Feb;36(2):e24213. doi: 10.1002/jcla.24213. Epub 2021 Dec 30. J Clin Lab Anal. 2022. PMID: 34970791 Free PMC article.
-
Current Advances in Basic and Translational Research of Cholangiocarcinoma.Cancers (Basel). 2021 Jul 1;13(13):3307. doi: 10.3390/cancers13133307. Cancers (Basel). 2021. PMID: 34282753 Free PMC article. Review.
-
miR-29b-3p Inhibitor Alleviates Hypomethylation-Related Aberrations Through a Feedback Loop Between miR-29b-3p and DNA Methylation in Cardiomyocytes.Front Cell Dev Biol. 2022 Apr 11;10:788799. doi: 10.3389/fcell.2022.788799. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35478963 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

