Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung
- PMID: 33597218
- PMCID: PMC7893659
- DOI: 10.1136/jitc-2020-001999
Real-world survival outcomes with immune checkpoint inhibitors in large-cell neuroendocrine tumors of lung
Abstract
Background: Little is known regarding the efficacy of immune checkpoint inhibitors (ICI) in patients with advanced large-cell neuroendocrine lung carcinoma (aLCNEC).
Methods: 125 consecutive patients with aLCNEC were identified in the electronic databases of 4 participating cancer centers. The patients were divided into group A (patients who received ICI, n=41) and group B (patients who did not receive ICI, n=84). Overall survival since advanced disease diagnosis (OS DX) and OS since ICI initiation (OS ICI) were captured.
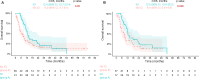
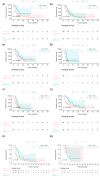
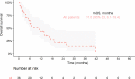
Results: With a median follow-up of 11.8 months (mo) (IQR 7.5-17.9) and 6.0mo (IQR 3.1-10.9), 66% and 76% of patients died in groups A and B, respectively. Median OS DX was 12.4mo (95% CI 10.7 to 23.4) and 6.0mo (95% CI 4.7 to 9.4) in groups A and B, respectively (log-rank test, p=0.02). For ICI administration, HR for OS DX was 0.59 (95% CI 0.38 to 0.93, p=0.02-unadjusted), and 0.58 (95% CI 0.34 to 0.98, p=0.04-adjusted for age, Eastern Cooperative Oncology Group (ECOG) performance status (PS), presence of liver metastases and chemotherapy administration). In a propensity score matching analysis (n=74; 37 patients in each group matched for age and ECOG PS), median OS DX was 12.5 mo (95% CI 10.6 to 25.2) and 8.4 mo (95% CI 5.4 to 16.9) in matched groups A and B, respectively (log-rank test, p=0.046). OS ICI for patients receiving ICI as monotherapy (n=36) was 11.0 mo (95% CI 6.1 to 19.4).
Conclusions: With the limitations of retrospective design and small sample size, the results of this real-world cohort analysis suggest a positive impact of ICI on OS in aLCNEC.
Keywords: active; immunotherapy; lung neoplasms; programmed cell death 1 receptor.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: ED reported consulting fees from MSD, BMS, Astra Zeneca, Roche, Boehringer Ingelheim, Pfizer, Novartis, Takeda. MM reported consulting fees from Boehringer Ingelheim, Roche, Astra Zeneca, MSD, BMS, Abbvie, Takeda, Pomicell. CK reported research funding (to institution) from AstraZeneca, BMS, Novartis, Regeneron, Tesaro, Karyopharm, Debiopharm, Jassen, and Mirati and consulting fees from Novartis. SVL reported consulting fees from AstraZeneca, Beigene, Blueprint, Boehringer Ingelheim, Bristol-Myers Squibb, Catalyst, Celgene, Clovis, Corvus, G1 Therapeutics, Genentech, Guardant Health, Inivata, Janssen, Jazz, Lilly, LOXO, MSD, PharmaMar, Pfizer, Regeneron, and Takeda and research grants (to institution) from Alkermes, AstraZeneca, Bayer, Blueprint, Bristol-Myers Squibb, Genentech, Lilly, Lycera, Merck, Molecular Partners, Pfizer, Rain Therapeutics, RAPT, Spectrum, and Turning Point Therapeutics. DU reported consulting fees and non-financial support from BMS, Astra Zeneca, Takeda, consulting fees MSD, Roche and Boehringer Ingelheim. AZ reported grants from BMS, personal fees from Roche, MSD, BMS, Astra Zeneca, Takeda. AO reported advisory fees from MSD, BMS, Roche, Astra Zeneca, Novartis, Boehringer Ingelheim. MW reported speaker fees and funding (to institution) from Roche, MSD. JB reported grants and personal fees from MSD, Roche, BMS, Abbvie, Pfizer, Novartis, Astra Zeneca, Boehringer Ingelheim, Takeda.
Figures
Similar articles
-
Efficacy of immune check-point inhibitors (ICPi) in large cell neuroendocrine tumors of lung (LCNEC).Lung Cancer. 2020 May;143:40-46. doi: 10.1016/j.lungcan.2020.03.008. Epub 2020 Mar 12. Lung Cancer. 2020. PMID: 32203769
-
Efficacy of immune checkpoint inhibitors in non-small cell lung cancer with uncommon histology: a propensity-score-matched analysis.BMC Pulm Med. 2021 Oct 2;21(1):309. doi: 10.1186/s12890-021-01681-6. BMC Pulm Med. 2021. PMID: 34600514 Free PMC article.
-
Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors : Antibiotics immune checkpoint inhibitors in advanced NSCLC.Cancer Chemother Pharmacol. 2020 Jan;85(1):121-131. doi: 10.1007/s00280-019-03993-1. Epub 2019 Nov 19. Cancer Chemother Pharmacol. 2020. PMID: 31745593
-
Clinical outcomes of non-small cell lung cancer patients with leptomeningeal metastases after immune checkpoint inhibitor treatments.Eur J Cancer. 2021 Jun;150:23-30. doi: 10.1016/j.ejca.2021.03.037. Epub 2021 Apr 18. Eur J Cancer. 2021. PMID: 33882375
-
The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression.Eur J Cancer. 2021 May;149:73-81. doi: 10.1016/j.ejca.2021.02.040. Epub 2021 Apr 7. Eur J Cancer. 2021. PMID: 33838391
Cited by
-
TEM8 in Oncogenesis: Protein Biology, Pre-Clinical Agents, and Clinical Rationale.Cells. 2023 Nov 14;12(22):2623. doi: 10.3390/cells12222623. Cells. 2023. PMID: 37998358 Free PMC article. Review.
-
Comprehensive Dissection of Treatment Patterns and Outcome for Patients With Metastatic Large-Cell Neuroendocrine Lung Carcinoma.Front Oncol. 2021 Jul 8;11:673901. doi: 10.3389/fonc.2021.673901. eCollection 2021. Front Oncol. 2021. PMID: 34307143 Free PMC article.
-
Pulmonary Large Cell Neuroendocrine Carcinoma.Pathol Oncol Res. 2022 Oct 11;28:1610730. doi: 10.3389/pore.2022.1610730. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36304941 Free PMC article. Review.
-
Immune Checkpoint Blockade in Lung Carcinoids with Aggressive Behaviour: One More Arrow in Our Quiver?J Clin Med. 2022 Feb 16;11(4):1019. doi: 10.3390/jcm11041019. J Clin Med. 2022. PMID: 35207291 Free PMC article. Review.
-
Complete and durable response of pulmonary large-cell neuroendocrine carcinoma to pembrolizumab.Cancer Rep (Hoboken). 2022 Aug;5(8):e1589. doi: 10.1002/cnr2.1589. Epub 2021 Nov 24. Cancer Rep (Hoboken). 2022. PMID: 34817132 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
