A 2-pyridone amide inhibitor of transcriptional activity in Chlamydia trachomatis
- PMID: 33593835
- PMCID: PMC8092867
- DOI: 10.1128/AAC.01826-20
A 2-pyridone amide inhibitor of transcriptional activity in Chlamydia trachomatis
Abstract
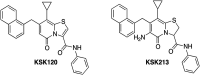
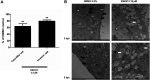
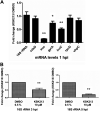
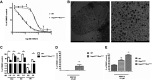
Chlamydia trachomatis is a strict intracellular bacterium that causes sexually transmitted infections and eye infections that can lead to life-long sequelae. Treatment options are limited to broad-spectrum antibiotics that disturb the commensal flora and contribute to selection of antibiotic-resistant bacteria. Hence, development of novel drugs that specifically target C. trachomatis would be beneficial. 2-pyridone amides are potent and specific inhibitors of Chlamydia infectivity. The first generation compound KSK120, inhibits the developmental cycle of Chlamydia resulting in reduced infectivity of progeny bacteria. Here, we show that the improved, highly potent second-generation 2-pyridone amide KSK213 allowed normal growth and development of C. trachomatis and the effect was only observable upon re-infection of new cells. Progeny elementary bodies (EBs) produced in the presence of KSK213 were unable to activate transcription of essential genes in early development and did not differentiate into the replicative form, the reticulate body (RB). The effect was specific to C. trachomatis since KSK213 was inactive in the closely related animal pathogen C. muridarum and in C. caviae The molecular target of KSK213 may thus be different in C. trachomatis or non-essential in C. muridarum and C. caviae Resistance to KSK213 was mediated by a combination of amino acid substitutions in both DEAD/DEAH RNA helicase and RNAse III, which may indicate inhibition of the transcriptional machinery as the mode of action. 2-pyridone amides provide a novel antibacterial strategy and starting points for development of highly specific drugs for C. trachomatis infections.
Copyright © 2021 American Society for Microbiology.
Figures


Similar articles
-
A 2-pyridone-amide inhibitor targets the glucose metabolism pathway of Chlamydia trachomatis.mBio. 2014 Dec 30;6(1):e02304-14. doi: 10.1128/mBio.02304-14. mBio. 2014. PMID: 25550323 Free PMC article.
-
Impact of Active Metabolism on Chlamydia trachomatis Elementary Body Transcript Profile and Infectivity.J Bacteriol. 2018 Jun 25;200(14):e00065-18. doi: 10.1128/JB.00065-18. Print 2018 Jul 15. J Bacteriol. 2018. PMID: 29735758 Free PMC article.
-
Thiazolino 2-Pyridone Amide Inhibitors of Chlamydia trachomatis Infectivity.J Med Chem. 2016 Mar 10;59(5):2094-108. doi: 10.1021/acs.jmedchem.5b01759. Epub 2016 Feb 22. J Med Chem. 2016. PMID: 26849778
-
Natural products for the treatment of trachoma and Chlamydia trachomatis.Molecules. 2015 Mar 5;20(3):4180-203. doi: 10.3390/molecules20034180. Molecules. 2015. PMID: 25751782 Free PMC article. Review.
-
Chlamydial plasmids and bacteriophages.Acta Biochim Pol. 2015;62(1):1-6. doi: 10.18388/abp.2014_764. Epub 2015 Feb 6. Acta Biochim Pol. 2015. PMID: 25654356 Review.
Cited by
-
Differential Effects of Small Molecule Inhibitors on the Intracellular Chlamydia Infection.mBio. 2022 Aug 30;13(4):e0107622. doi: 10.1128/mbio.01076-22. Epub 2022 Jun 15. mBio. 2022. PMID: 35703434 Free PMC article.
-
Fascinating Molecular and Immune Escape Mechanisms in the Treatment of STIs (Syphilis, Gonorrhea, Chlamydia, and Herpes Simplex).Int J Mol Sci. 2022 Mar 24;23(7):3550. doi: 10.3390/ijms23073550. Int J Mol Sci. 2022. PMID: 35408911 Free PMC article. Review.
-
Alternative strategies for Chlamydia treatment: Promising non-antibiotic approaches.Front Microbiol. 2022 Nov 23;13:987662. doi: 10.3389/fmicb.2022.987662. eCollection 2022. Front Microbiol. 2022. PMID: 36504792 Free PMC article. Review.
-
Therapeutic Options for Chlamydia trachomatis Infection: Present and Future.Antibiotics (Basel). 2022 Nov 16;11(11):1634. doi: 10.3390/antibiotics11111634. Antibiotics (Basel). 2022. PMID: 36421278 Free PMC article. Review.
References
-
- World Health Organization. 2012. Global incidence and prevalence of selected curable sexually transmitted infections—2008. World Health Organization, Geneva, Switzerland.
-
- Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi:10.1371/journal.pone.0143304. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

