Diagnostics for SARS-CoV-2 infections
- PMID: 33589798
- PMCID: PMC8264308
- DOI: 10.1038/s41563-020-00906-z
Diagnostics for SARS-CoV-2 infections
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread to nearly every corner of the globe, causing societal instability. The resultant coronavirus disease 2019 (COVID-19) leads to fever, sore throat, cough, chest and muscle pain, dyspnoea, confusion, anosmia, ageusia and headache. These can progress to life-threatening respiratory insufficiency, also affecting the heart, kidney, liver and nervous systems. The diagnosis of SARS-CoV-2 infection is often confused with that of influenza and seasonal upper respiratory tract viral infections. Due to available treatment strategies and required containments, rapid diagnosis is mandated. This Review brings clarity to the rapidly growing body of available and in-development diagnostic tests, including nanomaterial-based tools. It serves as a resource guide for scientists, physicians, students and the public at large.
Conflict of interest statement
Author declaration
The authors declare no competing interests.
Figures
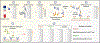

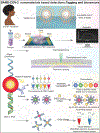
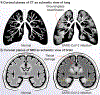
Comment in
-
Infection and Inflammation of Genitourinary Tract.J Urol. 2021 Jul;206(1):139-140. doi: 10.1097/JU.0000000000001812. Epub 2021 Apr 21. J Urol. 2021. PMID: 33881342 No abstract available.
Similar articles
-
The potential application of electrochemical biosensors in the COVID-19 pandemic: A perspective on the rapid diagnostics of SARS-CoV-2.Biosens Bioelectron. 2021 Mar 15;176:112905. doi: 10.1016/j.bios.2020.112905. Epub 2020 Dec 17. Biosens Bioelectron. 2021. PMID: 33358285 Free PMC article. Review.
-
Advancements in detection of SARS-CoV-2 infection for confronting COVID-19 pandemics.Lab Invest. 2022 Jan;102(1):4-13. doi: 10.1038/s41374-021-00663-w. Epub 2021 Sep 8. Lab Invest. 2022. PMID: 34497366 Free PMC article. Review.
-
Rapid SARS-CoV-2 antigen detection potentiates early diagnosis of COVID-19 disease.Biosci Trends. 2021 May 11;15(2):93-99. doi: 10.5582/bst.2021.01090. Epub 2021 Mar 26. Biosci Trends. 2021. PMID: 33776018
-
COVID-19 diagnosis -A review of current methods.Biosens Bioelectron. 2021 Jan 15;172:112752. doi: 10.1016/j.bios.2020.112752. Epub 2020 Oct 24. Biosens Bioelectron. 2021. PMID: 33126180 Free PMC article. Review.
-
Ravaging SARS-CoV-2: rudimentary diagnosis and puzzling immunological responses.Curr Med Res Opin. 2021 Feb;37(2):207-217. doi: 10.1080/03007995.2020.1862532. Epub 2020 Dec 26. Curr Med Res Opin. 2021. PMID: 33306409 Free PMC article. Review.
Cited by
-
Real-time identification of epistatic interactions in SARS-CoV-2 from large genome collections.Genome Biol. 2024 Aug 22;25(1):228. doi: 10.1186/s13059-024-03355-y. Genome Biol. 2024. PMID: 39175058 Free PMC article.
-
Peptide-based direct electrochemical detection of receptor binding domains of SARS-CoV-2 spike protein in pristine samples.Sens Actuators B Chem. 2023 Feb 15;377:133052. doi: 10.1016/j.snb.2022.133052. Epub 2022 Nov 23. Sens Actuators B Chem. 2023. PMID: 36438197 Free PMC article.
-
Immunologic mediators profile in COVID-19 convalescence.Sci Rep. 2024 Sep 9;14(1):20930. doi: 10.1038/s41598-024-71419-x. Sci Rep. 2024. PMID: 39251702 Free PMC article.
-
Multiplexed detection of viral antigen and RNA using nanopore sensing and encoded molecular probes.Nat Commun. 2023 Nov 14;14(1):7362. doi: 10.1038/s41467-023-43004-9. Nat Commun. 2023. PMID: 37963924 Free PMC article.
-
Psychological health, wellbeing and COVID-19: Comparing previously infected and non-infected South African employees.Front Psychol. 2022 Nov 3;13:1013377. doi: 10.3389/fpsyg.2022.1013377. eCollection 2022. Front Psychol. 2022. PMID: 36405203 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID-19. https://wwwcdcgov/coronavirus/2019-ncov/lab/guidelines-clinical-specimen... (2020).
-
- Wadman M, et al. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science (2020).
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- R01 NS097195/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- R01 AI158160/AI/NIAID NIH HHS/United States
- R01 MH121402-01A1/MH/NIMH NIH HHS/United States
- T32 NS105594/NS/NINDS NIH HHS/United States
- R01 NS033249/NS/NINDS NIH HHS/United States
- R01 AG043530/AG/NIA NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH121402P01/MH/NIMH NIH HHS/United States
- R01 MH121402/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

