Nucleolar stress in C9orf72 and sporadic ALS spinal motor neurons precedes TDP-43 mislocalization
- PMID: 33588953
- PMCID: PMC7885352
- DOI: 10.1186/s40478-021-01125-6
Nucleolar stress in C9orf72 and sporadic ALS spinal motor neurons precedes TDP-43 mislocalization
Abstract
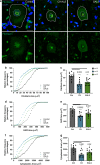
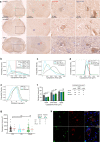
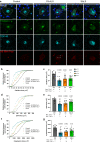
Nucleolar stress has been implicated in the pathology and disease pathogenesis of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) from repeat expansions of GGGGCC in C9orf72 (C9-ALS/FTLD) but not in sporadic ALS (SALS). Previously we reported that antisense RNA transcripts are unique in C9-ALS because of their nucleolar localization in spinal motor neurons and correlation with TDP-43 mislocalization, the hallmark proteinopathy of ALS and FTLD. Here we report our further studies of 11 SALS, 11 C9-ALS and 11 control spinal cords. We find that nucleolar stress manifests specifically as shrinkage in nucleoli of C9-ALS spinal motor neurons. Nucleolar size reduction is greatest in similarly sized alpha motor neurons from C9-ALS cases and results are not skewed by the number of surviving neurons from each ALS spinal cord. Surprisingly, nucleolar shrinkage occurs before main pathological hallmarks-TDP-43 mislocalization or antisense RNA foci-appear and this suggest that nucleolar stress can precede pathology in C9-ALS, findings previously identified in C9-FTLD using sense RNA foci and dipeptide repeat proteins as pathological markers. Importantly, these observations are also seen in SALS motor neurons and thus nucleolar stress appears to be a significant and probably upstream problem in sporadic disease.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Dipeptide repeat protein inclusions are rare in the spinal cord and almost absent from motor neurons in C9ORF72 mutant amyotrophic lateral sclerosis and are unlikely to cause their degeneration.Acta Neuropathol Commun. 2015 Jun 25;3:38. doi: 10.1186/s40478-015-0218-y. Acta Neuropathol Commun. 2015. PMID: 26108573 Free PMC article.
-
Antisense RNA foci in the motor neurons of C9ORF72-ALS patients are associated with TDP-43 proteinopathy.Acta Neuropathol. 2015 Jul;130(1):63-75. doi: 10.1007/s00401-015-1429-9. Epub 2015 May 6. Acta Neuropathol. 2015. PMID: 25943887 Free PMC article.
-
The RNA-binding motif 45 (RBM45) protein accumulates in inclusion bodies in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) patients.Acta Neuropathol. 2012 Nov;124(5):717-32. doi: 10.1007/s00401-012-1045-x. Epub 2012 Sep 21. Acta Neuropathol. 2012. PMID: 22993125 Free PMC article.
-
NEAT1 lncRNA and amyotrophic lateral sclerosis.Neurochem Int. 2021 Nov;150:105175. doi: 10.1016/j.neuint.2021.105175. Epub 2021 Sep 2. Neurochem Int. 2021. PMID: 34481908 Review.
-
C9orf72 isoforms in Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration.Brain Res. 2016 Sep 15;1647:43-49. doi: 10.1016/j.brainres.2016.04.062. Epub 2016 Apr 29. Brain Res. 2016. PMID: 27134035 Review.
Cited by
-
Nuclear α-Synuclein-Derived Cytotoxic Effect via Altered Ribosomal RNA Processing in Primary Mouse Embryonic Fibroblasts.Int J Mol Sci. 2023 Jan 21;24(3):2132. doi: 10.3390/ijms24032132. Int J Mol Sci. 2023. PMID: 36768455 Free PMC article.
-
Nucleolar stress controls mutant Huntington toxicity and monitors Huntington's disease progression.Cell Death Dis. 2021 Dec 8;12(12):1139. doi: 10.1038/s41419-021-04432-x. Cell Death Dis. 2021. PMID: 34880223 Free PMC article.
-
LINC complex alterations are a key feature of sporadic and familial ALS/FTD.Acta Neuropathol Commun. 2024 Apr 25;12(1):69. doi: 10.1186/s40478-024-01778-z. Acta Neuropathol Commun. 2024. PMID: 38664831 Free PMC article.
-
(Dis)Solving the problem of aberrant protein states.Dis Model Mech. 2021 May 1;14(5):dmm048983. doi: 10.1242/dmm.048983. Epub 2021 May 4. Dis Model Mech. 2021. PMID: 33942880 Free PMC article. Review.
-
Nucleolus and Nucleolar Stress: From Cell Fate Decision to Disease Development.Cells. 2022 Sep 27;11(19):3017. doi: 10.3390/cells11193017. Cells. 2022. PMID: 36230979 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

