Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis
- PMID: 33586297
- PMCID: PMC8017262
- DOI: 10.1111/1759-7714.13867
Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis
Abstract
Background: Due to the increasing number of trials with immune checkpoint inhibitors (ICIs) in the first-line therapy of non-small cell lung cancer (NSCLC) patients, we performed a systematic review and meta-analyses to investigate the difference between anti PD-1 and PD-L1 antibodies, used alone or in combination with chemotherapy, through adjusted indirect analysis to minimize the potential bias regarding overall survival (OS), progression-free survival (PFS), overall response rate (ORR) and grade 3-5 adverse events (AEs).
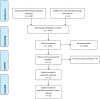
Methods: A systematic review of studies reporting clinical outcomes and toxicity associated with first-line therapy employing anti-PD1 or anti-PD-L1 antibodies alone, or in combination with chemotherapy, to treat metastatic, treatment-naïve NSCLC patients was performed. Primary outcomes were OS, PFS, ORR and grade 3-5 AEs. We used a random-effects model to generate pooled estimates for proportions. Meta-analyses using pooled risk ratios were performed for binary outcomes from comparative studies with the random effects model.
Results: A total of 13 eligible studies met our eligibility criteria, including 7673 patients. In the ICI-chemotherapy combination subgroup, we observed that anti-PD1 therapy was associated with better OS (p = 0.022) and PFS (p = 0.029) compared with anti-PD-L1 therapy. In the monotherapy subgroup, there was no statistical difference between the use of anti-PD-1 and anti-PD-L1 for OS and PFS. With regard to ORR and toxicity, in the ICI-chemotherapy combination subgroup, we observed a trend of better ORR (p = 0.12) with the use of anti-PD1 therapy and less frequent grade 3-5 AEs compared to the use of anti-PD-L1 therapy (p = 0.0302). In the monotherapy subgroup, there was no statistical difference between the use of anti-PD-1 and anti-PD-L1 regarding ORR and toxicity.
Conclusions: Our study suggests that PD-1 drug plus chemotherapy is superior to anti-PD-L1 plus chemotherapy for NSCLC; nevertheless, as monotherapy, both strategies appear to be similar.
Keywords: NSCLC; anti-PD-L1; anti-PD1; immunotherapy; meta-analysis.
© 2021 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures




Comment in
-
Anti-PD1 or anti-PD-L1 antibodies alone or in combination with chemotherapy first-line treatment of advanced non-small cell lung cancer.Thorac Cancer. 2022 Apr;13(7):1104-1105. doi: 10.1111/1759-7714.14358. Epub 2022 Feb 24. Thorac Cancer. 2022. PMID: 35199960 Free PMC article. No abstract available.
Similar articles
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Dec 14;12(12):CD013257. doi: 10.1002/14651858.CD013257.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 Apr 30;4:CD013257. doi: 10.1002/14651858.CD013257.pub3. PMID: 33316104 Free PMC article. Updated.
-
Single or combined immune checkpoint inhibitors compared to first-line platinum-based chemotherapy with or without bevacizumab for people with advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2021 Apr 30;4(4):CD013257. doi: 10.1002/14651858.CD013257.pub3. Cochrane Database Syst Rev. 2021. PMID: 33930176 Free PMC article.
-
Efficacy and safety of immune checkpoint inhibitors for advanced non-small cell lung cancer with or without PD-L1 selection: A systematic review and network meta-analysis.Chin Med J (Engl). 2023 Sep 20;136(18):2156-2165. doi: 10.1097/CM9.0000000000002750. Epub 2023 Aug 18. Chin Med J (Engl). 2023. PMID: 37596898 Free PMC article.
-
Benefits of combination therapy with immune checkpoint inhibitors and predictive role of tumour mutation burden in hepatocellular carcinoma: A systematic review and meta-analysis.Int Immunopharmacol. 2022 Nov;112:109244. doi: 10.1016/j.intimp.2022.109244. Epub 2022 Sep 18. Int Immunopharmacol. 2022. PMID: 36126410 Review.
-
Efficacy of PD-1/PD-L1 Inhibitors versus Chemotherapy in Lung Cancer with Brain Metastases: A Systematic Review and Meta-Analysis.J Immunol Res. 2022 May 20;2022:4518898. doi: 10.1155/2022/4518898. eCollection 2022. J Immunol Res. 2022. PMID: 35637793 Free PMC article. Review.
Cited by
-
Agonistic Bivalent Human scFvs-Fcγ Fusion Antibodies to OX40 Ectodomain Enhance T Cell Activities against Cancer.Vaccines (Basel). 2023 Dec 7;11(12):1826. doi: 10.3390/vaccines11121826. Vaccines (Basel). 2023. PMID: 38140230 Free PMC article.
-
PD-1/PD-L1 Inhibitors plus Chemotherapy Versus Chemotherapy Alone for Resectable Non-Small Cell Lung Cancer: A Systematic Review and Meta-Analysis of Randomized Controlled Trials.Cancers (Basel). 2023 Oct 26;15(21):5143. doi: 10.3390/cancers15215143. Cancers (Basel). 2023. PMID: 37958317 Free PMC article. Review.
-
Anti-PD-1 Monoclonal Antibodies (mAbs) Are Superior to Anti-PD-L1 mAbs When Combined with Chemotherapy in First-Line Treatment for Metastatic Non-Small Cell Lung Cancer (mNSCLC): A Network Meta-Analysis.Biomedicines. 2023 Jun 26;11(7):1827. doi: 10.3390/biomedicines11071827. Biomedicines. 2023. PMID: 37509467 Free PMC article.
-
Anti-PD1 or anti-PD-L1 antibodies alone or in combination with chemotherapy first-line treatment of advanced non-small cell lung cancer.Thorac Cancer. 2022 Apr;13(7):1104-1105. doi: 10.1111/1759-7714.14358. Epub 2022 Feb 24. Thorac Cancer. 2022. PMID: 35199960 Free PMC article. No abstract available.
-
Protein Binding Nanoparticles as an Integrated Platform for Cancer Diagnosis and Treatment.Adv Sci (Weinh). 2022 Oct;9(29):e2202453. doi: 10.1002/advs.202202453. Epub 2022 Aug 18. Adv Sci (Weinh). 2022. PMID: 35981878 Free PMC article.
References
-
- Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non‐small cell lung cancer: a review. JAMA. 2019;322(8):764–74. - PubMed
-
- Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17(11):1497–508. - PMC - PubMed
-
- Reck M, Rodríguez‐Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med. 2016;375(19):1823–33. - PubMed
-
- Barlesi F, Nishio M, Cobo M, Steele N, Paramonov V, Parente B, et al. IMpower132: efficacy of atezolizumab (atezo) + carboplatin (carbo)/cisplatin (cis) + pemetrexed (pem) as 1L treatment in key subgroups with stage IV non‐squamous non‐small cell lung cancer (NSCLC). European Society for Medical Oncology (ESMO) 2018 Congress; October 22, 2018; Munich, Germany 2018. http://bit.ly/2QD5DTD
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

