Natural infections with different Plasmodium species induce antibodies reactive to a chimeric Plasmodium vivax recombinant protein
- PMID: 33579292
- PMCID: PMC7880512
- DOI: 10.1186/s12936-021-03626-0
Natural infections with different Plasmodium species induce antibodies reactive to a chimeric Plasmodium vivax recombinant protein
Abstract
Background: As malaria incidence and transmission in a region decreases, it becomes increasingly difficult to identify areas of active transmission. Improved methods for identifying and monitoring foci of active malaria transmission are needed in areas of low parasite prevalence in order to achieve malaria elimination. Serological assays can provide population-level infection history to inform elimination campaigns.
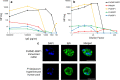
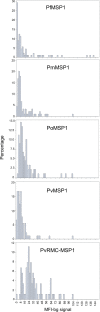
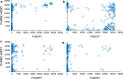
Methods: A bead-based multiplex antibody detection assay was used to evaluate a chimeric Plasmodium vivax MSP1 protein (PvRMC-MSP1), designed to be broadly immunogenic for use in vaccine studies, to act as a pan-malaria serological tool based on its ability to capture IgG in plasma samples obtained from naturally exposed individuals. Samples from 236 US travellers with PCR confirmed infection status from all four major Plasmodium species infecting humans, Plasmodium falciparum (n = 181), Plasmodium vivax (n = 38), Plasmodium malariae (n = 4), and Plasmodium ovale (n = 13) were tested for IgG capture using PvRMC-MSP1 as well as the four recombinant MSP1-19 kD isoforms representative of these Plasmodium species.
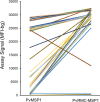
Results: Regardless of infecting Plasmodium species, a large proportion of plasma samples from infected US travellers provided a high assay signal to the PvRMC-MSP1 chimeric protein, with 115 high responders out of 236 samples assessed (48.7%). When grouped by active infection, 38.7% P. falciparum-, 92.1% of P. vivax-, 75.0% P. malariae-, and 53.4% of P. ovale-infected individuals displayed high assay signals in response to PvRMC-MSP1. It was also determined that plasma from P. vivax-infected individuals produced increased assay signals in response to the PvRMC-MSP1 chimera as compared to the recombinant PvMSP1 for 89.5% (34 out of 38) of individuals. PvRMC-MSP1 also showed improved ability to capture IgG antibodies from P. falciparum-infected individuals when compared to the capture by recombinant PvMSP1, with high assay signals observed for 38.7% of P. falciparum-infected travellers in response to PvRMC-MSP1 IgG capture compared to just 1.1% who were high responders to capture by the recombinant PvMSP1 protein.
Conclusions: These results support further study of designed antigens as an approach for increasing sensitivity or broadening binding capacity to improve existing serological tools for determining population-level exposure to Plasmodium species. Including both broad-reacting and Plasmodium species-specific antigen-coated beads in an assay panel could provide a nuanced view of population-level exposure histories, an extensive IgG profile, and detailed seroestimates. A more sensitive serological tool for detection of P. vivax exposure would aid malaria elimination campaigns in co-endemic areas and regions where P. vivax is the dominant parasite.
Keywords: Chimeric protein; Malaria; Multiplex; Plasmodium vivax; Seroepidemiology; Serology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
The use of a chimeric antigen for Plasmodium falciparum and P. vivax seroprevalence estimates from community surveys in Ethiopia and Costa Rica.PLoS One. 2022 May 25;17(5):e0263485. doi: 10.1371/journal.pone.0263485. eCollection 2022. PLoS One. 2022. PMID: 35613090 Free PMC article.
-
Specificity of the IgG antibody response to Plasmodium falciparum, Plasmodium vivax, Plasmodium malariae, and Plasmodium ovale MSP119 subunit proteins in multiplexed serologic assays.Malar J. 2018 Nov 9;17(1):417. doi: 10.1186/s12936-018-2566-0. Malar J. 2018. PMID: 30413163 Free PMC article.
-
A panel of recombinant proteins from human-infective Plasmodium species for serological surveillance.Malar J. 2020 Jan 17;19(1):31. doi: 10.1186/s12936-020-3111-5. Malar J. 2020. PMID: 31952523 Free PMC article.
-
Malaria Elimination: Time to Target All Species.Am J Trop Med Hyg. 2018 Jul;99(1):17-23. doi: 10.4269/ajtmh.17-0869. Epub 2018 May 10. Am J Trop Med Hyg. 2018. PMID: 29761762 Free PMC article. Review.
-
Non-falciparum malaria infections in Uganda, does it matter? A review of the published literature.Malar J. 2024 Jul 12;23(1):207. doi: 10.1186/s12936-024-05023-9. Malar J. 2024. PMID: 38997728 Free PMC article. Review.
Cited by
-
The use of a chimeric antigen for Plasmodium falciparum and P. vivax seroprevalence estimates from community surveys in Ethiopia and Costa Rica.PLoS One. 2022 May 25;17(5):e0263485. doi: 10.1371/journal.pone.0263485. eCollection 2022. PLoS One. 2022. PMID: 35613090 Free PMC article.
-
Using Serological Markers for the Surveillance of Plasmodium vivax Malaria: A Scoping Review.Pathogens. 2023 May 31;12(6):791. doi: 10.3390/pathogens12060791. Pathogens. 2023. PMID: 37375481 Free PMC article. Review.
-
Construction, Expression, and Evaluation of the Naturally Acquired Humoral Immune Response against Plasmodium vivax RMC-1, a Multistage Chimeric Protein.Int J Mol Sci. 2023 Jul 18;24(14):11571. doi: 10.3390/ijms241411571. Int J Mol Sci. 2023. PMID: 37511330 Free PMC article.
-
Antibody Profile Comparison against MSP1 Antigens of Multiple Plasmodium Species in Human Serum Samples from Two Different Brazilian Populations Using a Multiplex Serological Assay.Pathogens. 2021 Sep 4;10(9):1138. doi: 10.3390/pathogens10091138. Pathogens. 2021. PMID: 34578170 Free PMC article.
References
-
- WHO. World malaria report 2020. Geneva, World Health Organization, 2020.
-
- Geiger C, Agustar HK, Compaoré G, Coulibaly B, Sié A, Becher H, et al. Declining malaria parasite prevalence and trends of asymptomatic parasitaemia in a seasonal transmission setting in north-western Burkina Faso between 2000 and 2009–2012. Malar J. 2013;12:27. doi: 10.1186/1475-2875-12-27. - DOI - PMC - PubMed
-
- Harris I, Sharrock WW, Bain LM, Gray K-A, Bobogare A, Boaz L, et al. A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malar J. 2010;9:254. doi: 10.1186/1475-2875-9-254. - DOI - PMC - PubMed
-
- Niang M, Thiam LG, Sane R, Diagne N, Talla C, Doucoure S, et al. Substantial asymptomatic submicroscopic Plasmodium carriage during dry season in low transmission areas in Senegal: Implications for malaria control and elimination. PLoS ONE. 2017;12:e0182189. doi: 10.1371/journal.pone.0182189. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

