Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression
- PMID: 33574763
- PMCID: PMC7870719
- DOI: 10.3389/fphys.2020.514494
Osthole Alleviates Neointimal Hyperplasia in Balloon-Induced Arterial Wall Injury by Suppressing Vascular Smooth Muscle Cell Proliferation and Downregulating Cyclin D1/CDK4 and Cyclin E1/CDK2 Expression
Abstract
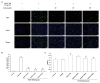
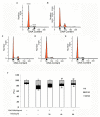
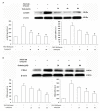
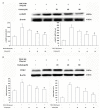
Percutaneous coronary intervention (PCI) is the most widely used therapy for treating ischemic heart disease. However, intimal hyperplasia and restenosis usually occur within months after angioplasty. Modern pharmacological researchers have proven that osthole, the major active coumarin of Cnidium monnieri (L.) Cusson, exerts potent antiproliferative effects in lung cancer cells, the human laryngeal cancer cell line RK33 and TE671 medulloblastoma cells, and its mechanism of action is related to cell cycle arrest. The goal of the present study was to observe the effect of osthole on vascular smooth muscle cell (VSMC) proliferation using platelet-derived growth factor-BB (PDGF-BB)-stimulated VSMCs isolated from rats and vascular balloon injury as models to further elucidate the molecular mechanisms underlying this activity. We detected the relative number of VSMCs by the MTT assay and EdU staining and examined cell cycle progression by flow cytometry. To more deeply probe the mechanisms, the protein expression levels of PCNA, the cyclin D1/CDK4 complex and the cyclin E1/CDK2 complex in balloon-treated rat carotid arteries and the mRNA and protein expression levels of the cyclin D1/CDK4 and cyclin E1/CDK2 complexes in VSMCs were detected by real-time RT-PCR and western blotting. The data showed that osthole significantly inhibited the proliferation of VSMCs induced by PDGF-BB. Furthermore, osthole caused apparent VSMC cycle arrest early in G0/G1 phase and decreased the expression of cyclin D1/CDK4 and cyclin E1/CDK2. Our results demonstrate that osthole can significantly inhibit PDGF-BB-induced VSMC proliferation and that its regulatory effects on cell cycle progression and proliferation may be related to the downregulation of cyclin D1/CDK4 and cyclin E1/CDK2 expression as well as the prevention of cell cycle progression from G0/G1 phase to S phase. The abovementioned mechanism may be responsible for the alleviation of neointimal hyperplasia in balloon-induced arterial wall injury by osthole.
Keywords: CDK2; CDK4; cyclin D1; cyclin E1; osthole; platelet-derived growth factor-BB; proliferation; vascular smooth muscle cells.
Copyright © 2021 Li, Li, Li, Lv, Gao, Li, Gong and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Osthole inhibits cell proliferation by regulating the TGF-β1/Smad/p38 signaling pathways in pulmonary arterial smooth muscle cells.Biomed Pharmacother. 2020 Jan;121:109640. doi: 10.1016/j.biopha.2019.109640. Epub 2019 Nov 25. Biomed Pharmacother. 2020. PMID: 31810114
-
Gambogic acid induces G0/G1 cell cycle arrest and cell migration inhibition via suppressing PDGF receptor β tyrosine phosphorylation and Rac1 activity in rat aortic smooth muscle cells.J Atheroscler Thromb. 2010 Sep 30;17(9):901-13. doi: 10.5551/jat.3491. Epub 2010 Jun 11. J Atheroscler Thromb. 2010. PMID: 20543524
-
Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury.Atherosclerosis. 2013 May;228(1):53-60. doi: 10.1016/j.atherosclerosis.2013.02.002. Epub 2013 Feb 18. Atherosclerosis. 2013. PMID: 23473423
-
Review on the protective activity of osthole against the pathogenesis of osteoporosis.Front Pharmacol. 2023 Aug 23;14:1236893. doi: 10.3389/fphar.2023.1236893. eCollection 2023. Front Pharmacol. 2023. PMID: 37680712 Free PMC article. Review.
-
Animal Models of Neointimal Hyperplasia and Restenosis: Species-Specific Differences and Implications for Translational Research.JACC Basic Transl Sci. 2021 Aug 11;6(11):900-917. doi: 10.1016/j.jacbts.2021.06.006. eCollection 2021 Nov. JACC Basic Transl Sci. 2021. PMID: 34869956 Free PMC article. Review.
Cited by
-
Icariside II Restores Vascular Smooth Muscle Cell Contractile Phenotype by Enhancing the Focal Adhesion Signaling Pathway in the Rat Vascular Remodeling Model.Front Pharmacol. 2022 Jun 13;13:897615. doi: 10.3389/fphar.2022.897615. eCollection 2022. Front Pharmacol. 2022. PMID: 35770073 Free PMC article.
-
Yohimbine Inhibits PDGF-Induced Vascular Smooth Muscle Cell Proliferation and Migration via FOXO3a Factor.Int J Mol Sci. 2024 Jun 24;25(13):6899. doi: 10.3390/ijms25136899. Int J Mol Sci. 2024. PMID: 39000009 Free PMC article.
-
Yohimbine, an α2-Adrenoceptor Antagonist, Suppresses PDGF-BB-Stimulated Vascular Smooth Muscle Cell Proliferation by Downregulating the PLCγ1 Signaling Pathway.Int J Mol Sci. 2022 Jul 21;23(14):8049. doi: 10.3390/ijms23148049. Int J Mol Sci. 2022. PMID: 35887391 Free PMC article.
References
-
- Begum N., Hockman S., Manganiello V. C. (2011). Phosphodiesterase 3A (PDE3A) deletion suppresses proliferation of cultured murine vascular smooth muscle cells (VSMCs) via inhibition of mitogen-activated protein kinase (MAPK) signaling and alterations in critical cell cycle regulatory proteins. J. Biol. Chem. 2011, 26238–26249. 10.1074/jbc.M110.214155, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

