Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs
- PMID: 33572566
- PMCID: PMC7866805
- DOI: 10.3390/ijms22031419
Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs
Abstract
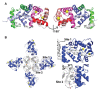
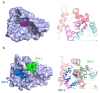
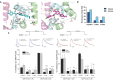
Ion channels are macromolecular complexes present in the plasma membrane and intracellular organelles of cells. Dysfunction of ion channels results in a group of disorders named channelopathies, which represent an extraordinary challenge for study and treatment. In this review, we will focus on voltage-gated potassium channels (KV), specifically on the KV4-family. The activation of these channels generates outward currents operating at subthreshold membrane potentials as recorded from myocardial cells (ITO, transient outward current) and from the somata of hippocampal neurons (ISA). In the heart, KV4 dysfunctions are related to Brugada syndrome, atrial fibrillation, hypertrophy, and heart failure. In hippocampus, KV4.x channelopathies are linked to schizophrenia, epilepsy, and Alzheimer's disease. KV4.x channels need to assemble with other accessory subunits (β) to fully reproduce the ITO and ISA currents. β Subunits affect channel gating and/or the traffic to the plasma membrane, and their dysfunctions may influence channel pharmacology. Among KV4 regulatory subunits, this review aims to analyze the KV4/KChIPs interaction and the effect of small molecule KChIP ligands in the A-type currents generated by the modulation of the KV4/KChIP channel complex. Knowledge gained from structural and functional studies using activators or inhibitors of the potassium current mediated by KV4/KChIPs will better help understand the underlying mechanism involving KV4-mediated-channelopathies, establishing the foundations for drug discovery, and hence their treatments.
Keywords: A-type current; KV4/KChIPs modulators; potassium channel interacting proteins (KChIPs); protein–protein interactions; transient outward current; voltage-gated potassium channels KV4.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


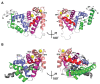

Similar articles
-
Distribution and functional expression of Kv4 family α subunits and associated KChIP β subunits in the bed nucleus of the stria terminalis.J Comp Neurol. 2014 Feb 15;522(3):609-25. doi: 10.1002/cne.23435. J Comp Neurol. 2014. PMID: 24037673 Free PMC article.
-
A potassium channel (Kv4) cloned from the heart of the tunicate Ciona intestinalis and its modulation by a KChIP subunit.J Exp Biol. 2006 Feb;209(Pt 4):731-47. doi: 10.1242/jeb.02032. J Exp Biol. 2006. PMID: 16449567
-
A polybasic motif in alternatively spliced KChIP2 isoforms prevents Ca2+ regulation of Kv4 channels.J Biol Chem. 2019 Mar 8;294(10):3683-3695. doi: 10.1074/jbc.RA118.006549. Epub 2019 Jan 8. J Biol Chem. 2019. PMID: 30622142 Free PMC article.
-
[Modulation of Kv4 channels by KChIPs clamping].Sheng Li Ke Xue Jin Zhan. 2009 Jan;40(1):9-13. Sheng Li Ke Xue Jin Zhan. 2009. PMID: 19408696 Review. Chinese.
-
Modulation by clamping: Kv4 and KChIP interactions.Neurochem Res. 2008 Oct;33(10):1964-9. doi: 10.1007/s11064-008-9705-x. Epub 2008 Apr 16. Neurochem Res. 2008. PMID: 18415675 Review.
Cited by
-
Modulation of KV4.3-KChIP2 Channels by IQM-266: Role of DPP6 and KCNE2.Int J Mol Sci. 2022 Aug 15;23(16):9170. doi: 10.3390/ijms23169170. Int J Mol Sci. 2022. PMID: 36012438 Free PMC article.
-
Design of Ultrapotent Genetically Encoded Inhibitors of Kv4.2 for Gating Neural Plasticity.J Neurosci. 2024 Feb 14;44(7):e2295222023. doi: 10.1523/JNEUROSCI.2295-22.2023. J Neurosci. 2024. PMID: 38154956 Free PMC article.
-
E3 ubiquitin ligase rififylin has yin and yang effects on rabbit cardiac transient outward potassium currents (Ito) and corresponding channel proteins.J Biol Chem. 2024 Mar;300(3):105759. doi: 10.1016/j.jbc.2024.105759. Epub 2024 Feb 15. J Biol Chem. 2024. PMID: 38367666 Free PMC article.
-
KV Channel-Interacting Proteins in the Neurological and Cardiovascular Systems: An Updated Review.Cells. 2023 Jul 20;12(14):1894. doi: 10.3390/cells12141894. Cells. 2023. PMID: 37508558 Free PMC article. Review.
-
Therapeutic role of voltage-gated potassium channels in age-related neurodegenerative diseases.Front Cell Neurosci. 2024 May 17;18:1406709. doi: 10.3389/fncel.2024.1406709. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38827782 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

