Retrograde and Anterograde Transport of Lat-Vesicles during the Immunological Synapse Formation: Defining the Finely-Tuned Mechanism
- PMID: 33572370
- PMCID: PMC7916135
- DOI: 10.3390/cells10020359
Retrograde and Anterograde Transport of Lat-Vesicles during the Immunological Synapse Formation: Defining the Finely-Tuned Mechanism
Abstract
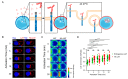
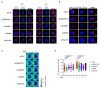
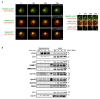
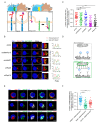
LAT is an important player of the signaling cascade induced by TCR activation. This adapter molecule is present at the plasma membrane of T lymphocytes and more abundantly in intracellular compartments. Upon T cell activation the intracellular pool of LAT is recruited to the immune synapse (IS). We previously described two pathways controlling LAT trafficking: retrograde transport from endosomes to the TGN, and anterograde traffic from the Golgi to the IS. We address the specific role of four proteins, the GTPase Rab6, the t-SNARE syntaxin-16, the v-SNARE VAMP7 and the golgin GMAP210, in each pathway. Using different methods (endocytosis and Golgi trap assays, confocal and TIRF microscopy, TCR-signalosome pull down) we show that syntaxin-16 is regulating the retrograde transport of LAT whereas VAMP7 is regulating the anterograde transport. Moreover, GMAP210 and Rab6, known to contribute to both pathways, are in our cellular context, specifically and respectively, involved in anterograde and retrograde transport of LAT. Altogether, our data describe how retrograde and anterograde pathways coordinate LAT enrichment at the IS and point to the Golgi as a central hub for the polarized recruitment of LAT to the IS. The role that this finely-tuned transport of signaling molecules plays in T-cell activation is discussed.
Keywords: LAT; T-cell activation; immune synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Rab6-dependent retrograde traffic of LAT controls immune synapse formation and T cell activation.J Exp Med. 2018 Apr 2;215(4):1245-1265. doi: 10.1084/jem.20162042. Epub 2018 Feb 12. J Exp Med. 2018. PMID: 29440364 Free PMC article.
-
Tethering of vesicles to the Golgi by GMAP210 controls LAT delivery to the immune synapse.Nat Commun. 2019 Jun 28;10(1):2864. doi: 10.1038/s41467-019-10891-w. Nat Commun. 2019. PMID: 31253807 Free PMC article.
-
VAMP7 controls T cell activation by regulating the recruitment and phosphorylation of vesicular Lat at TCR-activation sites.Nat Immunol. 2013 Jul;14(7):723-31. doi: 10.1038/ni.2609. Epub 2013 May 12. Nat Immunol. 2013. PMID: 23666293
-
T cell activation at the immunological synapse: vesicles emerge for LATer signaling.Sci Signal. 2010 May 11;3(121):pe16. doi: 10.1126/scisignal.3121pe16. Sci Signal. 2010. PMID: 20460646 Review.
-
Syntaxin 16's Newly Deciphered Roles in Autophagy.Cells. 2019 Dec 17;8(12):1655. doi: 10.3390/cells8121655. Cells. 2019. PMID: 31861136 Free PMC article. Review.
Cited by
-
F-Actin Dynamics in the Regulation of Endosomal Recycling and Immune Synapse Assembly.Front Cell Dev Biol. 2021 Jun 24;9:670882. doi: 10.3389/fcell.2021.670882. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34249926 Free PMC article. Review.
References
-
- Chemin K., Bohineust A., Dogniaux S., Tourret M., Guégan S., Miró-Mur F., Hivroz C. Cytokine Secretion by CD4+ T Cells at the Immunological Synapse Requires Cdc42-Dependent Local Actin Remodeling but Not Microtubule Organizing Center Polarity. J. Immunol. 2012;189:2159–2168. doi: 10.4049/jimmunol.1200156. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

