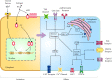
Estrogen receptors in pain modulation: cellular signaling
- PMID: 33568220
- PMCID: PMC7877067
- DOI: 10.1186/s13293-021-00364-5
Estrogen receptors in pain modulation: cellular signaling
Abstract
Sensory perception and emotional disorders are disproportionally represented in men and women and are thus thought to be modulated by different sex hormones in various conditions. Among the most important hormones perceived to affect sensory processing and transduction is estrogen. Numerous previous researchers have endeavored to demonstrate that estrogen is capable of modulating the activity of sensory neurons in peripheral and central sites in female, male, or castrated animals. However, the underlying mechanisms of its modulation of neuronal activity are somewhat unclear. In the present review, we discuss the possible cellular and molecular mechanisms involved in the modulation of nociception by estrogen.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Importance of sex to pain and its amelioration; relevance of spinal estrogens and its membrane receptors.Front Neuroendocrinol. 2012 Oct;33(4):412-24. doi: 10.1016/j.yfrne.2012.09.004. Epub 2012 Oct 2. Front Neuroendocrinol. 2012. PMID: 23036438 Free PMC article. Review.
-
Estrogenic influences in pain processing.Front Neuroendocrinol. 2013 Oct;34(4):329-49. doi: 10.1016/j.yfrne.2013.06.001. Epub 2013 Jun 29. Front Neuroendocrinol. 2013. PMID: 23817054 Review.
-
Neuroendocrine Mechanisms Governing Sex Differences in Hyperalgesic Priming Involve Prolactin Receptor Sensory Neuron Signaling.J Neurosci. 2020 Sep 9;40(37):7080-7090. doi: 10.1523/JNEUROSCI.1499-20.2020. Epub 2020 Aug 12. J Neurosci. 2020. PMID: 32801151 Free PMC article.
-
Neuroprotective and neurotoxic outcomes of androgens and estrogens in an oxidative stress environment.Biol Sex Differ. 2020 Mar 29;11(1):12. doi: 10.1186/s13293-020-0283-1. Biol Sex Differ. 2020. PMID: 32223745 Free PMC article.
-
Sex-specific modulation of spinal nociception by alpha2-adrenoceptors: differential regulation by estrogen and testosterone.Neuroscience. 2008 Jun 2;153(4):1268-77. doi: 10.1016/j.neuroscience.2008.03.008. Epub 2008 Mar 18. Neuroscience. 2008. PMID: 18434028 Free PMC article.
Cited by
-
Estrogen receptor β/substance P signaling in spinal cord mediates antinociceptive effect in a mouse model of discogenic low back pain.Front Cell Neurosci. 2023 Jan 23;16:1071012. doi: 10.3389/fncel.2022.1071012. eCollection 2022. Front Cell Neurosci. 2023. PMID: 36756381 Free PMC article.
-
Estrogenic impregnation alters pain expression: analysis through functional neuropeptidomics in a surgical rat model of osteoarthritis.Naunyn Schmiedebergs Arch Pharmacol. 2022 Jun;395(6):703-715. doi: 10.1007/s00210-022-02231-5. Epub 2022 Mar 23. Naunyn Schmiedebergs Arch Pharmacol. 2022. PMID: 35318491
-
Low back pain and osteoarthritis pain: a perspective of estrogen.Bone Res. 2023 Aug 4;11(1):42. doi: 10.1038/s41413-023-00280-x. Bone Res. 2023. PMID: 37542028 Free PMC article. Review.
-
The influence of sex on neuroimmune communication, pain, and physiology.Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review.
-
A graph-embedded topic model enables characterization of diverse pain phenotypes among UK biobank individuals.iScience. 2022 May 12;25(6):104390. doi: 10.1016/j.isci.2022.104390. eCollection 2022 Jun 17. iScience. 2022. PMID: 35637735 Free PMC article.
References
-
- Xiao X, et al. Estrogen in the anterior cingulate cortex contributes to pain-related aversion. Cerebral Cortex (New York, NY: 1991) 2013;23(9):2190–2203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical