Molecular Control of Oil Metabolism in the Endosperm of Seeds
- PMID: 33562710
- PMCID: PMC7915183
- DOI: 10.3390/ijms22041621
Molecular Control of Oil Metabolism in the Endosperm of Seeds
Abstract
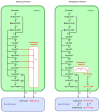
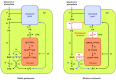
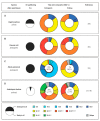
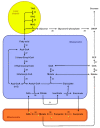
In angiosperm seeds, the endosperm develops to varying degrees and accumulates different types of storage compounds remobilized by the seedling during early post-germinative growth. Whereas the molecular mechanisms controlling the metabolism of starch and seed-storage proteins in the endosperm of cereal grains are relatively well characterized, the regulation of oil metabolism in the endosperm of developing and germinating oilseeds has received particular attention only more recently, thanks to the emergence and continuous improvement of analytical techniques allowing the evaluation, within a spatial context, of gene activity on one side, and lipid metabolism on the other side. These studies represent a fundamental step toward the elucidation of the molecular mechanisms governing oil metabolism in this particular tissue. In particular, they highlight the importance of endosperm-specific transcriptional controls for determining original oil compositions usually observed in this tissue. In the light of this research, the biological functions of oils stored in the endosperm of seeds then appear to be more diverse than simply constituting a source of carbon made available for the germinating seedling.
Keywords: endosperm; fatty acid; metabolism; oil; seed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Controlling lipid accumulation in cereal grains.Plant Sci. 2012 Apr;185-186:33-9. doi: 10.1016/j.plantsci.2011.09.002. Epub 2011 Sep 8. Plant Sci. 2012. PMID: 22325864
-
The overexpression of rice ACYL-CoA-BINDING PROTEIN2 increases grain size and bran oil content in transgenic rice.Plant J. 2019 Dec;100(6):1132-1147. doi: 10.1111/tpj.14503. Epub 2019 Sep 21. Plant J. 2019. PMID: 31437323
-
Transitions in wheat endosperm metabolism upon transcriptional induction of oil accumulation by oat endosperm WRINKLED1.BMC Plant Biol. 2020 May 25;20(1):235. doi: 10.1186/s12870-020-02438-9. BMC Plant Biol. 2020. PMID: 32450804 Free PMC article.
-
Endosperm development: dynamic processes and cellular innovations underlying sibling altruism.Wiley Interdiscip Rev Dev Biol. 2012 Jul-Aug;1(4):579-93. doi: 10.1002/wdev.31. Epub 2012 Feb 1. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801534 Review.
-
Regulators of Starch Biosynthesis in Cereal Crops.Molecules. 2021 Nov 24;26(23):7092. doi: 10.3390/molecules26237092. Molecules. 2021. PMID: 34885674 Free PMC article. Review.
Cited by
-
New Information of the Anatomy and Phytochemical Screening of Pentaclethra macroloba (Willd.) Kuntze (Caesalpinioideae-Leguminosae) Seeds.Int J Food Sci. 2023 Nov 29;2023:1446972. doi: 10.1155/2023/1446972. eCollection 2023. Int J Food Sci. 2023. PMID: 38075189 Free PMC article.
-
The Sunflower WRINKLED1 Transcription Factor Regulates Fatty Acid Biosynthesis Genes through an AW Box Binding Sequence with a Particular Base Bias.Plants (Basel). 2022 Apr 2;11(7):972. doi: 10.3390/plants11070972. Plants (Basel). 2022. PMID: 35406952 Free PMC article.
-
Identification of Two GDSL-Type Esterase/Lipase Genes Related to Tissue-Specific Lipolysis in Dendrobium catenatum by Multi-Omics Analysis.Life (Basel). 2022 Oct 9;12(10):1563. doi: 10.3390/life12101563. Life (Basel). 2022. PMID: 36294998 Free PMC article.
-
Degeneration of oil bodies by rough endoplasmic reticulum -associated protein during seed germination in Cannabis sativa.AoB Plants. 2023 Nov 22;15(6):plad082. doi: 10.1093/aobpla/plad082. eCollection 2023 Dec. AoB Plants. 2023. PMID: 38094511 Free PMC article.
-
Acyl-Acyl Carrier Protein Desaturases and Plant Biotic Interactions.Cells. 2021 Mar 18;10(3):674. doi: 10.3390/cells10030674. Cells. 2021. PMID: 33803674 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

