Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis
- PMID: 33553146
- PMCID: PMC7859330
- DOI: 10.3389/fcell.2020.607764
Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis
Abstract
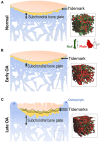
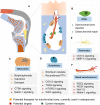
There is emerging awareness that subchondral bone remodeling plays an important role in the development of osteoarthritis (OA). This review presents recent investigations on the cellular and molecular mechanism of subchondral bone remodeling, and summarizes the current interventions and potential therapeutic targets related to OA subchondral bone remodeling. The first part of this review covers key cells and molecular mediators involved in subchondral bone remodeling (osteoclasts, osteoblasts, osteocytes, bone extracellular matrix, vascularization, nerve innervation, and related signaling pathways). The second part of this review describes candidate treatments for OA subchondral bone remodeling, including the use of bone-acting reagents and the application of regenerative therapies. Currently available clinical OA therapies and known responses in subchondral bone remodeling are summarized as a basis for the investigation of potential therapeutic mediators.
Keywords: cellular and molecular targets; osteoarthritis; regenerative therapy; stem cells; subchondral bone; subchondral bone remodeling.
Copyright © 2021 Zhu, Chan, Yung, Tuan and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Identification of soluble 14-3-3∊ as a novel subchondral bone mediator involved in cartilage degradation in osteoarthritis.Arthritis Rheum. 2013 Jul;65(7):1831-42. doi: 10.1002/art.37951. Arthritis Rheum. 2013. PMID: 23552998
-
Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain.J Clin Invest. 2019 Mar 1;129(3):1076-1093. doi: 10.1172/JCI121561. Epub 2019 Feb 4. J Clin Invest. 2019. PMID: 30530994 Free PMC article.
-
Strontium ranelate inhibits key factors affecting bone remodeling in human osteoarthritic subchondral bone osteoblasts.Bone. 2011 Sep;49(3):559-67. doi: 10.1016/j.bone.2011.06.005. Epub 2011 Jun 12. Bone. 2011. PMID: 21700005
-
Osteoarthritis year in review 2021: biology.Osteoarthritis Cartilage. 2022 Feb;30(2):207-215. doi: 10.1016/j.joca.2021.11.009. Epub 2021 Nov 18. Osteoarthritis Cartilage. 2022. PMID: 34801671 Review.
-
Do immune cells lead the way in subchondral bone disturbance in osteoarthritis?Prog Biophys Mol Biol. 2019 Nov;148:21-31. doi: 10.1016/j.pbiomolbio.2017.12.004. Epub 2017 Dec 22. Prog Biophys Mol Biol. 2019. PMID: 29277342 Review.
Cited by
-
Pathogenic Mechanisms and Therapeutic Approaches in Obesity-Related Knee Osteoarthritis.Biomedicines. 2023 Dec 20;12(1):9. doi: 10.3390/biomedicines12010009. Biomedicines. 2023. PMID: 38275369 Free PMC article. Review.
-
IgSF11 deficiency alleviates osteoarthritis in mice by suppressing early subchondral bone changes.Exp Mol Med. 2023 Dec;55(12):2576-2585. doi: 10.1038/s12276-023-01126-6. Epub 2023 Dec 1. Exp Mol Med. 2023. PMID: 38036734 Free PMC article.
-
Interplay between Inflammation and Pathological Bone Resorption: Insights into Recent Mechanisms and Pathways in Related Diseases for Future Perspectives.Int J Mol Sci. 2022 Feb 4;23(3):1786. doi: 10.3390/ijms23031786. Int J Mol Sci. 2022. PMID: 35163708 Free PMC article. Review.
-
Osteoarthritis Pathophysiology: Therapeutic Target Discovery may Require a Multifaceted Approach.Clin Geriatr Med. 2022 May;38(2):193-219. doi: 10.1016/j.cger.2021.11.015. Clin Geriatr Med. 2022. PMID: 35410676 Free PMC article. Review.
-
Vindoline Attenuates Osteoarthritis Progression Through Suppressing the NF-κB and ERK Pathways in Both Chondrocytes and Subchondral Osteoclasts.Front Pharmacol. 2022 Jan 12;12:764598. doi: 10.3389/fphar.2021.764598. eCollection 2021. Front Pharmacol. 2022. PMID: 35095488 Free PMC article.
References
-
- Behets C., Williams J. M., Chappard D., Devogelaer J. P., Manicourt D. H. (2004). Effects of calcitonin on subchondral trabecular bone changes and on osteoarthritic cartilage lesions after acute anterior cruciate ligament deficiency. J. Bone Miner. Res. 19, 1821–1826. 10.1359/JBMR.040609 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

