T Cells in Chronic Lymphocytic Leukemia: A Two-Edged Sword
- PMID: 33552073
- PMCID: PMC7857025
- DOI: 10.3389/fimmu.2020.612244
T Cells in Chronic Lymphocytic Leukemia: A Two-Edged Sword
Abstract
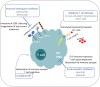
Chronic lymphocytic leukemia (CLL) is a malignancy of mature, antigen-experienced B lymphocytes. Despite great progress recently achieved in the management of CLL, the disease remains incurable, underscoring the need for further investigation into the underlying pathophysiology. Microenvironmental crosstalk has an established role in CLL pathogenesis and progression. Indeed, the malignant CLL cells are strongly dependent on interactions with other immune and non-immune cell populations that shape a highly orchestrated network, the tumor microenvironment (TME). The composition of the TME, as well as the bidirectional interactions between the malignant clone and the microenvironmental elements have been linked to disease heterogeneity. Mounting evidence implicates T cells present in the TME in the natural history of the CLL as well as in the establishment of certain CLL hallmarks e.g. tumor evasion and immune suppression. CLL is characterized by restrictions in the T cell receptor gene repertoire, T cell oligoclonal expansions, as well as shared T cell receptor clonotypes amongst patients, strongly alluding to selection by restricted antigenic elements of as yet undisclosed identity. Further, the T cells in CLL exhibit a distinctive phenotype with features of "exhaustion" likely as a result of chronic antigenic stimulation. This might be relevant to the fact that, despite increased numbers of oligoclonal T cells in the periphery, these cells are incapable of mounting effective anti-tumor immune responses, a feature perhaps also linked with the elevated numbers of T regulatory subpopulations. Alterations of T cell gene expression profile are associated with defects in both the cytoskeleton and immune synapse formation, and are generally induced by direct contact with the malignant clone. That said, these abnormalities appear to be reversible, which is why therapies targeting the T cell compartment represent a reasonable therapeutic option in CLL. Indeed, novel strategies, including CAR T cell immunotherapy, immune checkpoint blockade and immunomodulation, have come to the spotlight in an attempt to restore the functionality of T cells and enhance targeted cytotoxic activity against the malignant clone.
Keywords: T cell-based therapies; T lymphocytes; anti-tumor immunity; chronic lymphocytic leukemia; tumor microenvironment.
Copyright © 2021 Vlachonikola, Stamatopoulos and Chatzidimitriou.
Conflict of interest statement
KS and AC have received unrestricted grant support from Jannsen Pharmaceutica and Abbvie. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Immune Dysfunctions and Immune-Based Therapeutic Interventions in Chronic Lymphocytic Leukemia.Front Immunol. 2020 Nov 18;11:594556. doi: 10.3389/fimmu.2020.594556. eCollection 2020. Front Immunol. 2020. PMID: 33312177 Free PMC article. Review.
-
Improving therapy of chronic lymphocytic leukemia with chimeric antigen receptor T cells.Semin Oncol. 2016 Apr;43(2):291-9. doi: 10.1053/j.seminoncol.2016.02.006. Epub 2016 Feb 9. Semin Oncol. 2016. PMID: 27040708 Free PMC article. Review.
-
Control of PD-L1 expression in CLL-cells by stromal triggering of the Notch-c-Myc-EZH2 oncogenic signaling axis.J Immunother Cancer. 2021 Apr;9(4):e001889. doi: 10.1136/jitc-2020-001889. J Immunother Cancer. 2021. PMID: 33931470 Free PMC article.
-
Survival and Immunosuppression Induced by Hepatocyte Growth Factor in Chronic Lymphocytic Leukemia.Curr Mol Med. 2017;17(1):24-33. doi: 10.2174/1566524017666170220095838. Curr Mol Med. 2017. PMID: 28231754 Review.
-
T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers.Haematologica. 2017 Mar;102(3):562-572. doi: 10.3324/haematol.2016.151100. Epub 2016 Dec 7. Haematologica. 2017. PMID: 27927767 Free PMC article.
Cited by
-
Melanoma and CLL co-occurrence and survival: role of KC history.BMC Cancer. 2023 Nov 9;23(1):1084. doi: 10.1186/s12885-023-11573-z. BMC Cancer. 2023. PMID: 37946198 Free PMC article.
-
CD160 receptor in CLL: Current state and future avenues.Front Immunol. 2022 Nov 7;13:1028013. doi: 10.3389/fimmu.2022.1028013. eCollection 2022. Front Immunol. 2022. PMID: 36420268 Free PMC article. Review.
-
Activation and expansion of T-follicular helper cells in chronic lymphocytic leukemia nurselike cell co-cultures.Leukemia. 2022 May;36(5):1324-1335. doi: 10.1038/s41375-022-01519-y. Epub 2022 Feb 11. Leukemia. 2022. PMID: 35149845
-
The immunomodulatory molecule TIGIT is expressed by chronic lymphocytic leukemia cells and contributes to anergy.Haematologica. 2023 Aug 1;108(8):2101-2115. doi: 10.3324/haematol.2022.282177. Haematologica. 2023. PMID: 36655432 Free PMC article.
-
The Tumor Microenvironment in Tumorigenesis and Therapy Resistance Revisited.Cancers (Basel). 2023 Jan 6;15(2):376. doi: 10.3390/cancers15020376. Cancers (Basel). 2023. PMID: 36672326 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources