The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model
- PMID: 33551849
- PMCID: PMC7858671
- DOI: 10.3389/fphys.2020.629421
The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model
Abstract
Background: Upregulation of the two-pore-domain potassium channel TASK-1 (hK2 P 3.1) was recently described in patients suffering from atrial fibrillation (AF) and resulted in shortening of the atrial action potential. In the human heart, TASK-1 channels facilitate repolarization and are specifically expressed in the atria. In the present study, we tested the antiarrhythmic effects of the experimental ion channel inhibitor A293 that is highly affine for TASK-1 in a porcine large animal model of persistent AF.
Methods: Persistent AF was induced in German landrace pigs by right atrial burst stimulation via implanted pacemakers using a biofeedback algorithm over 14 days. Electrophysiological and echocardiographic investigations were performed before and after the pharmacological treatment period. A293 was intravenously administered once per day. After a treatment period of 14 days, atrial cardiomyocytes were isolated for patch clamp measurements of currents and atrial action potentials. Hemodynamic consequences of TASK-1 inhibition were measured upon acute A293 treatment.
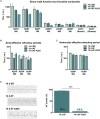
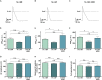
Results: In animals with persistent AF, the A293 treatment significantly reduced the AF burden (6.5% vs. 95%; P < 0.001). Intracardiac electrophysiological investigations showed that the atrial effective refractory period was prolonged in A293 treated study animals, whereas, the QRS width, QT interval, and ventricular effective refractory periods remained unchanged. A293 treatment reduced the upregulation of the TASK-1 current as well as the shortening of the action potential duration caused by AF. No central nervous side effects were observed. A mild but significant increase in pulmonary artery pressure was observed upon acute TASK-1 inhibition.
Conclusion: Pharmacological inhibition of atrial TASK-1 currents exerts in vivo antiarrhythmic effects that can be employed for rhythm control in a porcine model of persistent AF. Care has to be taken as TASK-1 inhibition may increase pulmonary artery pressure levels.
Keywords: A293; KCNK3; TASK-1; antiarrhythmic pharmacotherapy; atrial fibrillation; cardioversion.
Copyright © 2021 Wiedmann, Beyersdorf, Zhou, Kraft, Foerster, El-Battrawy, Lang, Borggrefe, Haefeli, Frey and Schmidt.
Conflict of interest statement
FW and CS have filed a patent application for KCNK3-based gene therapy for cardiac arrhythmia. FW, WH, and CS have filed a patent application for pharmacological TASK-1 inhibition in treatment of atrial arrhythmia. A293-d8 was kindly gifted by Sanofi-Aventis Deutschland GmbH (Frankfurt, Germany). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option.Front Pharmacol. 2021 Mar 4;12:638445. doi: 10.3389/fphar.2021.638445. eCollection 2021. Front Pharmacol. 2021. PMID: 33897427 Free PMC article. Review.
-
Pharmacologic TWIK-Related Acid-Sensitive K+ Channel (TASK-1) Potassium Channel Inhibitor A293 Facilitates Acute Cardioversion of Paroxysmal Atrial Fibrillation in a Porcine Large Animal Model.J Am Heart Assoc. 2020 May 18;9(10):e015751. doi: 10.1161/JAHA.119.015751. Epub 2020 May 9. J Am Heart Assoc. 2020. PMID: 32390491 Free PMC article.
-
Treatment of atrial fibrillation with doxapram: TASK-1 potassium channel inhibition as a novel pharmacological strategy.Cardiovasc Res. 2022 Jun 22;118(7):1728-1741. doi: 10.1093/cvr/cvab177. Cardiovasc Res. 2022. PMID: 34028533
-
Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K+ Channel-Related Acid-Sensitive K+ Channel-1) (K2P3.1) K+ Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling.Circ Arrhythm Electrophysiol. 2019 Sep;12(9):e007465. doi: 10.1161/CIRCEP.119.007465. Epub 2019 Sep 13. Circ Arrhythm Electrophysiol. 2019. PMID: 31514528
-
Unraveling the Role of K2P Channels in Atrial Fibrillation.Front Biosci (Schol Ed). 2022 Nov 22;14(4):31. doi: 10.31083/j.fbs1404031. Front Biosci (Schol Ed). 2022. PMID: 36575841 Review.
Cited by
-
Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option.Front Pharmacol. 2021 Mar 4;12:638445. doi: 10.3389/fphar.2021.638445. eCollection 2021. Front Pharmacol. 2021. PMID: 33897427 Free PMC article. Review.
-
Two-Pore-Domain Potassium (K2P-) Channels: Cardiac Expression Patterns and Disease-Specific Remodelling Processes.Cells. 2021 Oct 27;10(11):2914. doi: 10.3390/cells10112914. Cells. 2021. PMID: 34831137 Free PMC article. Review.
-
Exploring the involvement of TASK-1 in the control of isolated rat right atrium function from healthy animals and an experimental model of monocrotaline-induced pulmonary hypertension.Naunyn Schmiedebergs Arch Pharmacol. 2023 Dec;396(12):3775-3788. doi: 10.1007/s00210-023-02569-4. Epub 2023 Jun 20. Naunyn Schmiedebergs Arch Pharmacol. 2023. PMID: 37338577
-
Simultaneous Quantification and Pharmacokinetic Characterization of Doxapram and 2-Ketodoxapram in Porcine Plasma and Brain Tissue.Pharmaceutics. 2022 Mar 31;14(4):762. doi: 10.3390/pharmaceutics14040762. Pharmaceutics. 2022. PMID: 35456597 Free PMC article.
-
Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients.Int J Mol Sci. 2023 Aug 16;24(16):12859. doi: 10.3390/ijms241612859. Int J Mol Sci. 2023. PMID: 37629037 Free PMC article. Review.
References
-
- Bazett H. C. (1920). An analysis of time relations of electrocardiograms. Heart 7 353–367.
LinkOut - more resources
Full Text Sources
Other Literature Sources

