Non-cell autonomous astrocyte-mediated neuronal toxicity in prion diseases
- PMID: 33546775
- PMCID: PMC7866439
- DOI: 10.1186/s40478-021-01123-8
Non-cell autonomous astrocyte-mediated neuronal toxicity in prion diseases
Abstract
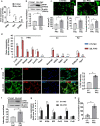
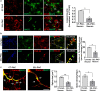
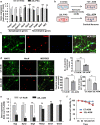
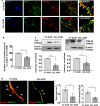
Under normal conditions, astrocytes perform a number of important physiological functions centered around neuronal support and synapse maintenance. In neurodegenerative diseases including Alzheimer's, Parkinson's and prion diseases, astrocytes acquire reactive phenotypes, which are sustained throughout the disease progression. It is not known whether in the reactive states associated with prion diseases, astrocytes lose their ability to perform physiological functions and whether the reactive states are neurotoxic or, on the contrary, neuroprotective. The current work addresses these questions by testing the effects of reactive astrocytes isolated from prion-infected C57BL/6J mice on primary neuronal cultures. We found that astrocytes isolated at the clinical stage of the disease exhibited reactive, pro-inflammatory phenotype, which also showed downregulation of genes involved in neurogenic and synaptogenic functions. In astrocyte-neuron co-cultures, astrocytes from prion-infected animals impaired neuronal growth, dendritic spine development and synapse maturation. Toward examining the role of factors secreted by reactive astrocytes, astrocyte-conditioned media was found to have detrimental effects on neuronal viability and synaptogenic functions via impairing synapse integrity, and by reducing spine size and density. Reactive microglia isolated from prion-infected animals were found to induce phenotypic changes in primary astrocytes reminiscent to those observed in prion-infected mice. In particular, astrocytes cultured with reactive microglia-conditioned media displayed hypertrophic morphology and a downregulation of genes involved in neurogenic and synaptogenic functions. In summary, the current study provided experimental support toward the non-cell autonomous mechanisms behind neurotoxicity in prion diseases and demonstrated that the astrocyte reactive phenotype associated with prion diseases is synaptotoxic.
Keywords: Astrocytes; Microglia; Neuroinflammation; Prion diseases; Prions; Synaptic toxicity.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures
Similar articles
-
Reactive astrocytes associated with prion disease impair the blood brain barrier.Neurobiol Dis. 2023 Sep;185:106264. doi: 10.1016/j.nbd.2023.106264. Epub 2023 Aug 18. Neurobiol Dis. 2023. PMID: 37597815 Free PMC article.
-
Phagocytic Activities of Reactive Microglia and Astrocytes Associated with Prion Diseases Are Dysregulated in Opposite Directions.Cells. 2021 Jul 8;10(7):1728. doi: 10.3390/cells10071728. Cells. 2021. PMID: 34359897 Free PMC article.
-
RNA-seq and network analysis reveal unique glial gene expression signatures during prion infection.Mol Brain. 2020 May 7;13(1):71. doi: 10.1186/s13041-020-00610-8. Mol Brain. 2020. PMID: 32381108 Free PMC article.
-
On the reactive states of astrocytes in prion diseases.Prion. 2021 Dec;15(1):87-93. doi: 10.1080/19336896.2021.1930852. Prion. 2021. PMID: 34057026 Free PMC article. Review.
-
Neuroinflammation, Microglia, and Cell-Association during Prion Disease.Viruses. 2019 Jan 15;11(1):65. doi: 10.3390/v11010065. Viruses. 2019. PMID: 30650564 Free PMC article. Review.
Cited by
-
Isoflurane Disrupts Postsynaptic Density-95 Protein Interactions Causing Neuronal Synapse Loss and Cognitive Impairment in Juvenile Mice via Canonical NO-mediated Protein Kinase-G Signaling.Anesthesiology. 2022 Aug 1;137(2):212-231. doi: 10.1097/ALN.0000000000004264. Anesthesiology. 2022. PMID: 35504002 Free PMC article.
-
Reactive astrocytes in prion diseases: Friend or foe?PLoS Pathog. 2024 Jun 20;20(6):e1012286. doi: 10.1371/journal.ppat.1012286. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38900746 Free PMC article. Review. No abstract available.
-
Reactive astrocytes associated with prion disease impair the blood brain barrier.Neurobiol Dis. 2023 Sep;185:106264. doi: 10.1016/j.nbd.2023.106264. Epub 2023 Aug 18. Neurobiol Dis. 2023. PMID: 37597815 Free PMC article.
-
Astrocyte in prion disease: a double-edged sword.Neural Regen Res. 2022 Aug;17(8):1659-1665. doi: 10.4103/1673-5374.332202. Neural Regen Res. 2022. PMID: 35017412 Free PMC article. Review.
-
Strain-Dependent Morphology of Reactive Astrocytes in Human- and Animal-Vole-Adapted Prions.Biomolecules. 2023 Apr 27;13(5):757. doi: 10.3390/biom13050757. Biomolecules. 2023. PMID: 37238627 Free PMC article.
References
-
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, et al. A Unique microglia type associated with restricting development of Alzheimer’s disease. Cell. 2017;169(7):1276–1290.e1217. doi: 10.1016/j.cell.2017.05.018. - DOI - PubMed
-
- Chen W-T, Lu A, Craessaerts K, Pavie B, Sala Frigerio C, Mancuso R, Qian X, Lalakova J, Kühnemund M, Voytyuk I et al Spatial and temporal transcriptomics reveal microglia-astroglia crosstalk in the amyloid-β plaque cell niche of Alzheimer’s disease. bioRxiv 2019:719930.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

