Cell-density independent increased lymphocyte production and loss rates post-autologous HSCT
- PMID: 33538246
- PMCID: PMC7886352
- DOI: 10.7554/eLife.59775
Cell-density independent increased lymphocyte production and loss rates post-autologous HSCT
Abstract
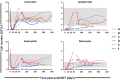
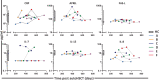
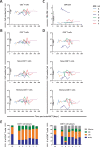
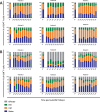
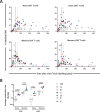
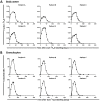
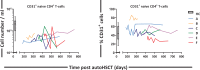
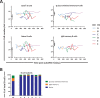
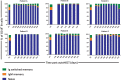
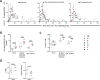
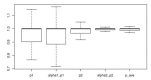
Lymphocyte numbers need to be quite tightly regulated. It is generally assumed that lymphocyte production and lifespan increase homeostatically when lymphocyte numbers are low and, vice versa, return to normal once cell numbers have normalized. This widely accepted concept is largely based on experiments in mice, but is hardly investigated in vivo in humans. Here we quantified lymphocyte production and loss rates in vivo in patients 0.5-1 year after their autologous hematopoietic stem cell transplantation (autoHSCT). We indeed found that the production rates of most T- and B-cell subsets in autoHSCT-patients were two to eight times higher than in healthy controls, but went hand in hand with a threefold to ninefold increase in cell loss rates. Both rates also did not normalize when cell numbers did. This shows that increased lymphocyte production and loss rates occur even long after autoHSCT and can persist in the face of apparently normal cell numbers.
Keywords: autologous hematopoietic; deuterium labeling; human; immunology; inflammation; lymphocyte dynamics; lymphopenia; mathematical modeling; stem cell transplantation.
© 2021, Baliu-Piqué et al.
Conflict of interest statement
MB, Vv, JD, Lv, AJ, SO, Mv, Rd, JB, KT No competing interests declared, JK reports grants from Novartis, Miltenyi Biotech, and Gadeta. Is inventor on multiple patents dealing with γδ T-cell research, ligands, and isolation techniques, and is scientific co-founder and shareholder of Gadeta. (Patent number: 9546998, 9891211, 10324083, 10578609. Publication number: 20200368278, 20200363397, 20190271688, 2019020961, 201901692603, 20180188234, 20170319674, 20170174741, 20150050670).
Figures










Similar articles
-
Immune reconstitution after autologous hematopoietic stem cell transplantation in relation to underlying disease, type of high-dose therapy and infectious complications.Haematologica. 2000 Aug;85(8):832-8. Haematologica. 2000. PMID: 10942930
-
Higher infused CD34+ hematopoietic stem cell dose correlates with earlier lymphocyte recovery and better clinical outcome after autologous stem cell transplantation in non-Hodgkin's lymphoma.Transfusion. 2009 Sep;49(9):1890-900. doi: 10.1111/j.1537-2995.2009.02202.x. Epub 2009 May 11. Transfusion. 2009. PMID: 19453991
-
Evolution of peripheral blood T lymphocyte subsets after allogenic or autologous hematopoietic stem cell transplantation.Immunobiology. 2014 Aug;219(8):611-8. doi: 10.1016/j.imbio.2014.03.012. Epub 2014 Mar 21. Immunobiology. 2014. PMID: 24721705
-
A Case of Multiple Sclerosis-Like Relapsing Remitting Encephalomyelitis Following Allogeneic Hematopoietic Stem Cell Transplantation and a Review of the Published Literature.Front Immunol. 2020 May 5;11:668. doi: 10.3389/fimmu.2020.00668. eCollection 2020. Front Immunol. 2020. PMID: 32431694 Free PMC article. Review.
-
[STATE OF DEVELOPMENT OF HEMATOPOIETIC STEM CELL TRANSPLANTATION IN THE EUROPE AND WORLD].Lik Sprava. 2014 Jul-Aug;(7-8):117-21. Lik Sprava. 2014. PMID: 26118095 Review. Ukrainian.
Cited by
-
Quantifying cellular dynamics in mice using a novel fluorescent division reporter system.Front Immunol. 2023 Jul 27;14:1157705. doi: 10.3389/fimmu.2023.1157705. eCollection 2023. Front Immunol. 2023. PMID: 37575229 Free PMC article.
-
Are homeostatic mechanisms aiding the reconstitution of the T-cell pool during lymphopenia in humans?Front Immunol. 2022 Nov 22;13:1059481. doi: 10.3389/fimmu.2022.1059481. eCollection 2022. Front Immunol. 2022. PMID: 36483556 Free PMC article. Review.
-
Effect of cellular aging on memory T-cell homeostasis.Front Immunol. 2022 Aug 8;13:947242. doi: 10.3389/fimmu.2022.947242. eCollection 2022. Front Immunol. 2022. PMID: 36059495 Free PMC article.
-
Towards a unified model of naive T cell dynamics across the lifespan.Elife. 2022 Jun 9;11:e78168. doi: 10.7554/eLife.78168. Elife. 2022. PMID: 35678373 Free PMC article.
References
-
- Alho AC, Kim HT, Chammas MJ, Reynolds CG, Matos TR, Forcade E, Whangbo J, Nikiforow S, Cutler CS, Koreth J, Ho VT, Armand P, Antin JH, Alyea EP, Lacerda JF, Soiffer RJ, Ritz J. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127:646–657. doi: 10.1182/blood-2015-10-672345. - DOI - PMC - PubMed
-
- Avanzini MA, Locatelli F, Dos Santos C, Maccario R, Lenta E, Oliveri M, Giebel S, De Stefano P, Rossi F, Giorgiani G, Amendola G, Telli S, Marconi M. B lymphocyte reconstitution after hematopoietic stem cell transplantation: functional immaturity and slow recovery of memory CD27+ B cells. Experimental Hematology. 2005;33:480–486. doi: 10.1016/j.exphem.2005.01.005. - DOI - PubMed
-
- Bell EB, Sparshott SM, Drayson MT, Ford WL. The stable and permanent expansion of functional T lymphocytes in athymic nude rats after a single injection of mature T cells. The Journal of Immunology. 1987;139:1379–1384. - PubMed
-
- Bemark M, Holmqvist J, Abrahamsson J, Mellgren K. Translational Mini-Review series on B cell subsets in disease reconstitution after haematopoietic stem cell transplantation - revelation of B cell developmental pathways and lineage phenotypes. Clinical & Experimental Immunology. 2012;167:15–25. doi: 10.1111/j.1365-2249.2011.04469.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

