Inhibition of Bone Morphogenetic Protein 2 Suppresses the Stemness Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma via the MAPK/ERK Pathway
- PMID: 33536785
- PMCID: PMC7850411
- DOI: 10.2147/CMAR.S281969
Inhibition of Bone Morphogenetic Protein 2 Suppresses the Stemness Maintenance of Cancer Stem Cells in Hepatocellular Carcinoma via the MAPK/ERK Pathway
Abstract
Background: Hepatocellular carcinoma (HCC) remains a life-threatening malignant tumor. Cancer stem cells (CSCs) harbor tumor-initiating capacity and can be used as a therapeutic target for human malignancies. Bone morphogenetic proteins (BMPs) play a regulatory role in CSCs. This study investigated the role and mechanism of BMP2 in CSCs in HCC.
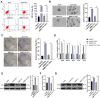
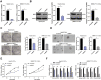
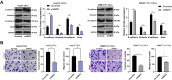
Methods: BMP2 expression in HCC tissues and cells, and CSCs from HepG2 cells and SMMC7721 cells (HepG2-CSCs and SMMC7721-CSCs) was measured. The association between BMP2 expression and prognosis of HCC patients was analyzed. CSCs were interfered with BMP2 to evaluate the abilities of colony and tumor sphere formation, levels of stemness-related markers, epithelial-mesenchymal transition (EMT), and invasion and migration. Levels of MAPK/ERK pathway-related proteins in HepG2-CSCs were detected after BMP2 knockdown. The effect of the activated MAPK/ERK pathway on HepG2-CSCs was assessed. Finally, the effect of BMP2 inhibition on CSCs in HCC was verified in vivo.
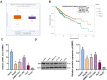
Results: BMP2 showed obvious upregulation in HCC tissues and cells and was further upregulated in CSCs in HCC, with its higher expression indicative of worse prognosis. Silencing BMP2 inhibited colony and tumor sphere formation, levels of stemness-related markers, as well as EMT, invasion and migration of HepG2-CSCs and SMMC7721-CSCs. The MAPK/ERK pathway was suppressed after BMP2 knockdown, and its activation reversed the inhibitory effect of shBMP2 on hepatic CSCs. BMP2 accelerated tumor growth and EMT of CSCs in HCC in vivo.
Conclusion: We concluded that BMP2 knockdown inhibited the EMT, proliferation and invasion of CSCs in HCC, thereby hindering the stemness maintenance via suppressing the MAPK/ERK pathway.
Keywords: MAPK/ERK pathway; bone morphogenetic protein 2; cancer stem cells; epithelial-mesenchymal transition; hepatocellular carcinoma; stemness.
© 2021 Guo et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest for this work.
Figures

Similar articles
-
BMP2 secretion from hepatocellular carcinoma cell HepG2 enhances angiogenesis and tumor growth in endothelial cells via activation of the MAPK/p38 signaling pathway.Stem Cell Res Ther. 2019 Aug 6;10(1):237. doi: 10.1186/s13287-019-1301-2. Stem Cell Res Ther. 2019. Retraction in: Stem Cell Res Ther. 2022 Apr 08;13(1):154. doi: 10.1186/s13287-022-02841-z PMID: 31387619 Free PMC article. Retracted.
-
ZnAs@SiO2 nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling.Theranostics. 2019 Jun 9;9(15):4391-4408. doi: 10.7150/thno.32462. eCollection 2019. Theranostics. 2019. PMID: 31285768 Free PMC article.
-
HOXB7 accelerates the malignant progression of hepatocellular carcinoma by promoting stemness and epithelial-mesenchymal transition.J Exp Clin Cancer Res. 2017 Jun 24;36(1):86. doi: 10.1186/s13046-017-0559-4. J Exp Clin Cancer Res. 2017. PMID: 28646927 Free PMC article.
-
BMP2 as a promising anticancer approach: functions and molecular mechanisms.Invest New Drugs. 2022 Dec;40(6):1322-1332. doi: 10.1007/s10637-022-01298-4. Epub 2022 Aug 30. Invest New Drugs. 2022. PMID: 36040572 Review.
-
MAPK/ERK Signaling Pathway in Hepatocellular Carcinoma.Cancers (Basel). 2021 Jun 17;13(12):3026. doi: 10.3390/cancers13123026. Cancers (Basel). 2021. PMID: 34204242 Free PMC article. Review.
Cited by
-
Association between the expression levels of ADAMTS16 and BMP2 and tumor budding in hepatocellular carcinoma.Oncol Lett. 2023 Apr 27;25(6):256. doi: 10.3892/ol.2023.13842. eCollection 2023 Jun. Oncol Lett. 2023. PMID: 37205917 Free PMC article.
-
Insight into the mechanisms regulating liver cancer stem cells by hepatitis B virus X protein.Infect Agent Cancer. 2024 Nov 11;19(1):56. doi: 10.1186/s13027-024-00618-y. Infect Agent Cancer. 2024. PMID: 39529119 Free PMC article. Review.
-
Altered BMP2/4 Signaling in Stem Cells and Their Niche: Different Cancers but Similar Mechanisms, the Example of Myeloid Leukemia and Breast Cancer.Front Cell Dev Biol. 2022 Jan 3;9:787989. doi: 10.3389/fcell.2021.787989. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35047500 Free PMC article. Review.
-
Mesenchymal stem cell-derived exosomes block malignant behaviors of hepatocellular carcinoma stem cells through a lncRNA C5orf66-AS1/microRNA-127-3p/DUSP1/ERK axis.Hum Cell. 2021 Nov;34(6):1812-1829. doi: 10.1007/s13577-021-00599-9. Epub 2021 Aug 24. Hum Cell. 2021. PMID: 34431063
-
Bone Morphogenetic Protein Signaling in Cancer; Some Topics in the Recent 10 Years.Front Cell Dev Biol. 2022 May 25;10:883523. doi: 10.3389/fcell.2022.883523. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35693928 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

