Physcion Protects Rats Against Cerebral Ischemia-Reperfusion Injury via Inhibition of TLR4/NF-kB Signaling Pathway
- PMID: 33536742
- PMCID: PMC7847770
- DOI: 10.2147/DDDT.S267856
Physcion Protects Rats Against Cerebral Ischemia-Reperfusion Injury via Inhibition of TLR4/NF-kB Signaling Pathway
Abstract
Background: Ischemic stroke (IS) is characterized by the rapid loss of brain function due to ischemia. Physcion has been found to have a neuroprotective effect against cerebral ischemia-reperfusion (I/R) injury. However, the mechanism by which physcion regulates cerebral I/R injury remains largely unknown.
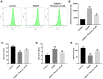
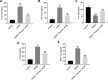
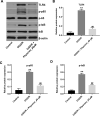
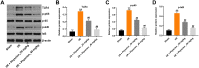
Methods: An oxygen-glucose deprivation/reperfusion (OGD/R) model in SH-SY5Y cells and a rat cerebral ischemia-reperfusion (I/R) model were established, respectively. CCK-8 and flow cytometry assays were used to detect the viability and apoptosis of SH-SY5Y cells. Moreover, enzyme-linked immunosorbent assay (ELISA) was used to measure the levels of SOD, MDA, GSH-Px, TNF-α, IL-1β, IL-6 and IL-10 in the supernatant of SH-SY5Y cells. Meanwhile, Western blot assay was used to detect the expressions of TLR4, p-p65 and p-IκB in SH-SY5Y cells and I/R rats.
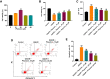
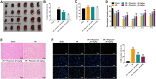
Results: In this study, physcion treatment significantly rescued OGD/R-induced neuronal injury. In addition, physcion decreased inflammatory response in SH-SY5Y cells after OGD/R insult, as shown by the decreased levels of the pro-inflammatory factors TNF-α, IL-1β, IL-6 and IL-10. Moreover, physcion attenuated the oxidative stress in OGD/R-treated SY-SY5Y cells, as evidenced by the increased SOD and GSH levels and the decreased ROS and MDA levels. Meanwhile, physcion significantly reduced cerebral infarction, attenuated neuronal injury and apoptosis in I/R rats. Furthermore, physcion markedly decreased the expressions of TLR4, p-NF-κB p65 and p-IκB in the brain tissues of rats subjected to I/R and in SH-SY5Y cells exposed to OGD/R.
Conclusion: In conclusion, our study indicated that physcion protected neuron cells against I/R injury in vitro and in vivo by inhibition of the TLR4/NF-kB pathway; thus, physcion might serve as a promising therapeutic candidate for IS.
Keywords: NF-κB; ischemia-reperfusion injury; physcion; stroke.
© 2021 Dong et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
Similar articles
-
Ginkgo diterpene lactones inhibit cerebral ischemia/reperfusion induced inflammatory response in astrocytes via TLR4/NF-κB pathway in rats.J Ethnopharmacol. 2020 Mar 1;249:112365. doi: 10.1016/j.jep.2019.112365. Epub 2019 Oct 31. J Ethnopharmacol. 2020. PMID: 31678414
-
[Acacetin protects rats from cerebral ischemia-reperfusion injury by regulating TLR4/NLRP3 signaling pathway].Zhongguo Zhong Yao Za Zhi. 2023 Nov;48(22):6107-6114. doi: 10.19540/j.cnki.cjcmm.20230719.401. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 38114218 Chinese.
-
MiR-155-5p accelerates cerebral ischemia-reperfusion injury via targeting DUSP14 by regulating NF-κB and MAPKs signaling pathways.Eur Rev Med Pharmacol Sci. 2020 Feb;24(3):1408-1419. doi: 10.26355/eurrev_202002_20198. Eur Rev Med Pharmacol Sci. 2020. PMID: 32096190
-
Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway.Neurochem Int. 2023 Jul;167:105553. doi: 10.1016/j.neuint.2023.105553. Epub 2023 May 23. Neurochem Int. 2023. PMID: 37230196 Review.
-
Loureirin B protects against cerebral ischemia/reperfusion injury through modulating M1/M2 microglial polarization via STAT6 / NF-kappaB signaling pathway.Eur J Pharmacol. 2023 Aug 15;953:175860. doi: 10.1016/j.ejphar.2023.175860. Epub 2023 Jun 16. Eur J Pharmacol. 2023. PMID: 37331681 Review.
Cited by
-
Fissistigma oldhamii (Hemsl.) Merr.: Ethnomedicinal, Phytochemistry, and Pharmacological Aspects.Plants (Basel). 2023 Dec 7;12(24):4094. doi: 10.3390/plants12244094. Plants (Basel). 2023. PMID: 38140421 Free PMC article. Review.
-
Exosomes-Mediated Signaling Pathway: A New Direction for Treatment of Organ Ischemia-Reperfusion Injury.Biomedicines. 2024 Feb 2;12(2):353. doi: 10.3390/biomedicines12020353. Biomedicines. 2024. PMID: 38397955 Free PMC article. Review.
-
Neuronal Responses to Ischemia: Scoping Review of Insights from Human-Derived In Vitro Models.Cell Mol Neurobiol. 2023 Oct;43(7):3137-3160. doi: 10.1007/s10571-023-01368-y. Epub 2023 Jun 28. Cell Mol Neurobiol. 2023. PMID: 37380886 Free PMC article.
-
Saposhnikoviae Radix Enhanced the Angiogenic and Anti-Inflammatory Effects of Huangqi Chifeng Tang in a Rat Model of Cerebral Infarction.Evid Based Complement Alternat Med. 2021 Sep 21;2021:4232708. doi: 10.1155/2021/4232708. eCollection 2021. Evid Based Complement Alternat Med. 2021. PMID: 34594389 Free PMC article.
-
Neuroprotective effect of Bouvardia ternifolia (Cav.) Schltdl via inhibition of TLR4/NF-κB, caspase-3/Bax/Bcl-2 pathways in ischemia/reperfusion injury in rats.Front Pharmacol. 2024 Sep 23;15:1471542. doi: 10.3389/fphar.2024.1471542. eCollection 2024. Front Pharmacol. 2024. PMID: 39376599 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

