Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges
- PMID: 33522005
- PMCID: PMC8247932
- DOI: 10.1002/med.21789
Adipose-derived stromal cells for nonhealing wounds: Emerging opportunities and challenges
Abstract
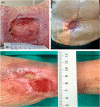
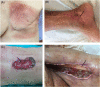
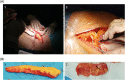
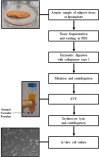
Wound healing complications affect thousands of people each year, thus constituting a profound economic and medical burden. Chronic wounds are a highly complex problem that usually affects elderly patients as well as patients with comorbidities such as diabetes, cancer (surgery, radiotherapy/chemotherapy) or autoimmune diseases. Currently available methods of their treatment are not fully effective, so new solutions are constantly being sought. Cell-based therapies seem to have great potential for use in stimulating wound healing. In recent years, much effort has been focused on characterizing of adipose-derived mesenchymal stromal cells (AD-MSCs) and evaluating their clinical use in regenerative medicine and other medical fields. These cells are easily obtained in large amounts from adipose tissue and show a high proregenerative potential, mainly through paracrine activities. In this review, the process of healing acute and nonhealing (chronic) wounds is detailed, with a special attention paid to the wounds of patients with diabetes and cancer. In addition, the methods and technical aspects of AD-MSCs isolation, culture and transplantation in chronic wounds are described, and the characteristics, genetic stability and role of AD-MSCs in wound healing are also summarized. The biological properties of AD-MSCs isolated from subcutaneous and visceral adipose tissue are compared. Additionally, methods to increase their therapeutic potential as well as factors that may affect their biological functions are summarized. Finally, their therapeutic potential in the treatment of diabetic and oncological wounds is also discussed.
Keywords: SVF; adipose-derived stromal cells; chronic wounds; diabetic ulcers; fat transfer; oncological wounds; wound healing.
© 2021 The Authors. Medicinal Research Reviews published by Wiley Periodicals LLC.
Figures

Similar articles
-
Adipose-Derived Mesenchymal Stromal Cells in Regenerative Medicine: State of Play, Current Clinical Trials, and Future Prospects.Adv Wound Care (New Rochelle). 2021 Jan;10(1):24-48. doi: 10.1089/wound.2020.1175. Epub 2020 Jun 2. Adv Wound Care (New Rochelle). 2021. PMID: 32470315 Free PMC article. Review.
-
Decellularized silk fibroin scaffold primed with adipose mesenchymal stromal cells improves wound healing in diabetic mice.Stem Cell Res Ther. 2014 Jan 14;5(1):7. doi: 10.1186/scrt396. Stem Cell Res Ther. 2014. PMID: 24423450 Free PMC article.
-
Effectiveness of preconditioned adipose-derived mesenchymal stem cells with photobiomodulation for the treatment of diabetic foot ulcers: a systematic review.Lasers Med Sci. 2022 Apr;37(3):1415-1425. doi: 10.1007/s10103-021-03451-6. Epub 2021 Oct 26. Lasers Med Sci. 2022. PMID: 34697696 Review.
-
Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects.Int J Mol Sci. 2021 Feb 3;22(4):1538. doi: 10.3390/ijms22041538. Int J Mol Sci. 2021. PMID: 33546464 Free PMC article.
-
Adipose Tissue-Derived Stromal Cells for Wound Healing.Adv Exp Med Biol. 2018;1119:133-149. doi: 10.1007/5584_2018_220. Adv Exp Med Biol. 2018. PMID: 29858972
Cited by
-
A Closed-system Technology for Mechanical Isolation of High Quantities of Stromal Vascular Fraction from Fat for Immediate Clinical Use.Plast Reconstr Surg Glob Open. 2023 Jun 22;11(6):e5096. doi: 10.1097/GOX.0000000000005096. eCollection 2023 Jun. Plast Reconstr Surg Glob Open. 2023. PMID: 37361510 Free PMC article.
-
Multilineage-Differentiating Stress-Enduring Cells (Muse Cells): An Easily Accessible, Pluripotent Stem Cell Niche with Unique and Powerful Properties for Multiple Regenerative Medicine Applications.Biomedicines. 2023 May 30;11(6):1587. doi: 10.3390/biomedicines11061587. Biomedicines. 2023. PMID: 37371682 Free PMC article. Review.
-
Fibroblast growth factor 1 ameliorates thin endometrium in rats through activation of the autophagic pathway.Front Pharmacol. 2023 Apr 20;14:1143096. doi: 10.3389/fphar.2023.1143096. eCollection 2023. Front Pharmacol. 2023. PMID: 37153783 Free PMC article.
-
Mesenchymal Stem Cells in Burn Wound Management.Int J Mol Sci. 2022 Dec 5;23(23):15339. doi: 10.3390/ijms232315339. Int J Mol Sci. 2022. PMID: 36499664 Free PMC article. Review.
-
Insights into the role of adipose-derived stem cells and secretome: potential biology and clinical applications in hypertrophic scarring.Stem Cell Res Ther. 2024 May 12;15(1):137. doi: 10.1186/s13287-024-03749-6. Stem Cell Res Ther. 2024. PMID: 38735979 Free PMC article. Review.
References
-
- Pikuła M, Langa P, Kosikowska P, Trzonkowski P. Stem cells and growth factors in wound healing. Postepy Hig Med Dosw. 2015;69:874‐885. - PubMed
-
- Reinke JM, Sorg H. Wound repair and regeneration. Eur Surg Res. 2012;49(1):35‐43. - PubMed
-
- Velnar T, Bailey T, Smrkolj V. The wound healing process: an overview of the cellular and molecular mechanisms. J Int Med Res. 2009;37(5):1528‐1542. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

