Addressing Non-linear System Dynamics of Single-Strand RNA Virus-Host Interaction
- PMID: 33519741
- PMCID: PMC7843927
- DOI: 10.3389/fmicb.2020.600254
Addressing Non-linear System Dynamics of Single-Strand RNA Virus-Host Interaction
Abstract
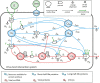
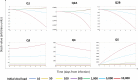
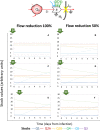
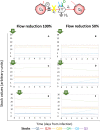
Positive single-strand ribonucleic acid [(+)ssRNA] viruses can cause multiple outbreaks, for which comprehensive tailored therapeutic strategies are still missing. Virus and host cell dynamics are tightly connected, generating a complex dynamics that conveys in virion assembly to ensure virus spread in the body. Starting from the knowledge of relevant processes in (+ss)RNA virus replication, transcription, translation, virions budding and shedding, and their respective energy costs, we built up a systems thinking (ST)-based diagram of the virus-host interaction, comprehensive of stocks, flows, and processes as well-described in literature. In ST approach, stocks and flows are expressed by a proxy of the energy embedded and transmitted, respectively, whereas processes are referred to the energy required for the system functioning. In this perspective, healthiness is just a particular configuration, in which stocks relevant for the system (equivalent but not limited to proteins, RNA, DNA, and all metabolites required for the survival) are constant, and the system behavior is stationary. At time of infection, the presence of additional stocks (e.g., viral protein and RNA and all metabolites required for virion assembly and spread) confers a complex network of feedbacks leading to new configurations, which can evolve to maximize the virions stock, thus changing the system structure, output, and purpose. The dynamic trajectories will evolve to achieve a new stationary status, a phenomenon described in microbiology as integration and symbiosis when the system is resilient enough to the changes, or the system may stop functioning and die. Application of external driving forces, acting on processes, can affect the dynamic trajectories adding a further degree of complexity, which can be captured by ST approach, used to address these new configurations. Investigation of system configurations in response to external driving forces acting is developed by computational analysis based on ST diagrams, with the aim at designing novel therapeutic approaches.
Keywords: RNA-virus; dynamics; evolution trajectories; modeling; simulation – computers; systems thinking (ST); virus–host interaction.
Copyright © 2021 Romano, Casazza and Gonella.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Mathematical modeling of plus-strand RNA virus replication to identify broad-spectrum antiviral treatment strategies.bioRxiv [Preprint]. 2022 Jul 25:2022.07.25.501353. doi: 10.1101/2022.07.25.501353. bioRxiv. 2022. Update in: PLoS Comput Biol. 2023 Apr 4;19(4):e1010423. doi: 10.1371/journal.pcbi.1010423 PMID: 35923314 Free PMC article. Updated. Preprint.
-
Macromolecular crowding: chemistry and physics meet biology (Ascona, Switzerland, 10-14 June 2012).Phys Biol. 2013 Aug;10(4):040301. doi: 10.1088/1478-3975/10/4/040301. Epub 2013 Aug 2. Phys Biol. 2013. PMID: 23912807
-
Planning Implications Related to Sterilization-Sensitive Science Investigations Associated with Mars Sample Return (MSR).Astrobiology. 2022 Jun;22(S1):S112-S164. doi: 10.1089/AST.2021.0113. Epub 2022 May 19. Astrobiology. 2022. PMID: 34904892
-
Imaging, Tracking and Computational Analyses of Virus Entry and Egress with the Cytoskeleton.Viruses. 2018 Mar 31;10(4):166. doi: 10.3390/v10040166. Viruses. 2018. PMID: 29614729 Free PMC article. Review.
-
[The great virus comeback].Biol Aujourdhui. 2013;207(3):153-68. doi: 10.1051/jbio/2013018. Epub 2013 Dec 13. Biol Aujourdhui. 2013. PMID: 24330969 Review. French.
Cited by
-
Using Stock-Flow Diagrams to Visualize Theranostic Approaches to Solid Tumors in Personalized Nanomedicine.Front Bioeng Biotechnol. 2021 Jul 22;9:709727. doi: 10.3389/fbioe.2021.709727. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 34368102 Free PMC article.
-
Modeling cell populations metabolism and competition under maximum power constraints.PLoS Comput Biol. 2023 Nov 8;19(11):e1011607. doi: 10.1371/journal.pcbi.1011607. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 37939139 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

