Combination gene therapy for HIV using a conditional suicidal gene with CCR5 knockout
- PMID: 33516234
- PMCID: PMC7847599
- DOI: 10.1186/s12985-021-01501-7
Combination gene therapy for HIV using a conditional suicidal gene with CCR5 knockout
Abstract
Background: Gene therapy approaches using hematopoietic stem cells to generate an HIV resistant immune system have been shown to be successful. The deletion of HIV co-receptor CCR5 remains a viable strategy although co-receptor switching to CXCR4 remains a major pitfall. To overcome this, we designed a dual gene therapy strategy that incorporates a conditional suicide gene and CCR5 knockout (KO) to overcome the limitations of CCR5 KO alone.
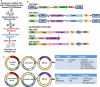
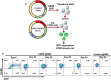
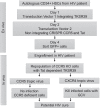
Methods: A two-vector system was designed that included an integrating lentiviral vector that expresses a HIV Tat dependent Thymidine Kinase mutant SR39 (TK-SR39) and GFP reporter gene. The second non-integrating lentiviral (NIL) vector expresses a CCR5gRNA-CRISPR/Cas9 cassette and HIV Tat protein.
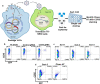
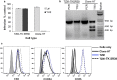
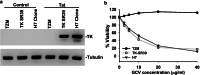
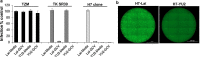
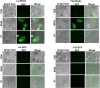
Results: Transduction of cells sequentially with the integrating followed by the NIL vector allows for insertion of the conditional suicide gene, KO of CCR5 and transient expression of GFP to enrich the modified cells. We used this strategy to modify TZM cells and generate a cell line that was resistant to CCR5 tropic viruses while permitting infection of CXCR4 tropic viruses which could be controlled via treatment with Ganciclovir.
Conclusions: Our study demonstrates proof of principle that a combination gene therapy for HIV is a viable strategy and can overcome the limitation of editing CCR5 gene alone.
Keywords: CCR5; CRISPR; CXCR4; Conditional; Cytotoxic; Ganciclovir; Gene therapy; HIV; HIV cure; TK-SR39.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

Similar articles
-
Conditional Cytotoxic Anti-HIV Gene Therapy for Selectable Cell Modification.Hum Gene Ther. 2016 May;27(5):400-15. doi: 10.1089/hum.2015.126. Epub 2016 Mar 30. Hum Gene Ther. 2016. PMID: 26800572 Free PMC article.
-
Simultaneous Knockout of CXCR4 and CCR5 Genes in CD4+ T Cells via CRISPR/Cas9 Confers Resistance to Both X4- and R5-Tropic Human Immunodeficiency Virus Type 1 Infection.Hum Gene Ther. 2018 Jan;29(1):51-67. doi: 10.1089/hum.2017.032. Epub 2017 Jun 9. Hum Gene Ther. 2018. PMID: 28599597
-
A simultaneous knockout knockin genome editing strategy in HSPCs potently inhibits CCR5- and CXCR4-tropic HIV-1 infection.Cell Stem Cell. 2024 Apr 4;31(4):499-518.e6. doi: 10.1016/j.stem.2024.03.002. Cell Stem Cell. 2024. PMID: 38579682
-
CCR5Δ32 mutation and HIV infection: basis for curative HIV therapy.Curr Opin Virol. 2015 Oct;14:24-9. doi: 10.1016/j.coviro.2015.06.007. Epub 2015 Jul 1. Curr Opin Virol. 2015. PMID: 26143158 Review.
-
Designer Nucleases: Gene-Editing Therapies using CCR5 as an Emerging Target in HIV.Curr HIV Res. 2019;17(5):306-323. doi: 10.2174/1570162X17666191025112918. Curr HIV Res. 2019. PMID: 31652113 Review.
Cited by
-
Targeting CCR5 as a Component of an HIV-1 Therapeutic Strategy.Front Immunol. 2022 Jan 20;12:816515. doi: 10.3389/fimmu.2021.816515. eCollection 2021. Front Immunol. 2022. PMID: 35126374 Free PMC article. Review.
-
Non-Integrating Lentiviral Vectors in Clinical Applications: A Glance Through.Biomedicines. 2022 Jan 5;10(1):107. doi: 10.3390/biomedicines10010107. Biomedicines. 2022. PMID: 35052787 Free PMC article. Review.
-
Antiretrovirals to CCR5 CRISPR/Cas9 gene editing - A paradigm shift chasing an HIV cure.Clin Immunol. 2023 Oct;255:109741. doi: 10.1016/j.clim.2023.109741. Epub 2023 Aug 21. Clin Immunol. 2023. PMID: 37611838 Free PMC article. Review.
-
Tat-dependent conditionally replicating adenoviruses expressing diphtheria toxin A for specifically killing HIV-1-infected cells.Mol Ther. 2024 Jul 3;32(7):2316-2327. doi: 10.1016/j.ymthe.2024.05.015. Epub 2024 May 11. Mol Ther. 2024. PMID: 38734901
-
Strategies for HIV-1 suppression through key genes and cell therapy.Front Med (Lausanne). 2023 Nov 29;10:1259995. doi: 10.3389/fmed.2023.1259995. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38093984 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

