Preventing or controlling periodontitis reduces the occurrence of osteonecrosis of the jaw (ONJ) in rice rats (Oryzomys palustris)
- PMID: 33515777
- PMCID: PMC8265021
- DOI: 10.1016/j.bone.2021.115866
Preventing or controlling periodontitis reduces the occurrence of osteonecrosis of the jaw (ONJ) in rice rats (Oryzomys palustris)
Abstract
Introduction: Osteonecrosis of the jaw (ONJ) is an adverse event that requires association of both systemic risk factors, such as powerful anti-resorptives (pARs; e.g. zoledronic acid [ZOL]), and local oral risk factors (e.g. tooth extraction, periodontitis). Whereas optimal oral health prior to initiate pARs is recognized as critically important for minimizing ONJ risk, the efficacy of preventive/maintenance measures in patients who are taking pARs is understudied. Rice rats fed a standard diet (STD), rich in insoluble fiber, develop localized periodontitis. STD-rats with localized periodontitis treated with ZOL for 18-24 wk develop ONJ. Hence, we hypothesized that controlling/preventing localized periodontitis in the ZOL-treated rats, reduces ONJ occurrence.
Methods: We used two approaches to attempt reducing periodontitis prevalence: 1) periodontal cleaning (PC); and 2) replacing the STD-diet with a nutritionally-equivalent diet high in soluble fiber (SF). 75 four-week-old male rats were weight-randomized into five groups (n = 15) in a 24-week experiment. Three groups ate the STD-diet and two the high SF-diet. STD-diet groups received intravenous (IV) vehicle (VEH) q4wks (STD + VEH), 80 μg/kg ZOL q4wks IV (STD + ZOL), or ZOL plus PC q2wks (STD + ZOL + PC). The SF-diet groups received VEH (SF + VEH) or ZOL (SF + ZOL). Jaws were processed for histopathology and evaluated for ONJ prevalence and tissue-level periodontitis.
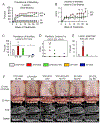
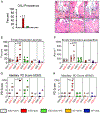
Results: 1) 40% of STD + VEH rats developed maxillary localized periodontitis with no ONJ; 2) 50% of STD + ZOL rats developed ONJ; 3) 7% of STD + ZOL + PC rats developed ONJ (p < 0.01 vs. STD + ZOL); and 4) one SF + ZOL rat developed localized periodontitis, and no SF + VEH or SF + ZOL rats developed ONJ (p < 0.001 vs. STD + ZOL).
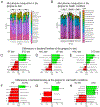
Conclusions: 1) Periodontal cleaning in ZOL-treated rats decreases localized periodontitis severity and reduces ONJ prevalence; and 2) feeding a SF-diet to ZOL-treated rats reduces both incidence of localized periodontitis and ONJ. Our data indicates strong oral microbial community shifts according to oral health condition and trends in the shifts associated with diet.
Keywords: Controlling; ONJ; Oral microbiome; Periodontitis; Prevention; Rice rats.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest
The authors have no conflicts of interest. AVA is a scientific advisory board member for Second Genome, Inc., which has not contributed to this research.
Figures

Similar articles
-
Anti-vascular endothelial growth factor antibody monotherapy causes destructive advanced periodontitis in rice rats (Oryzomys palustris).Bone. 2020 Jan;130:115141. doi: 10.1016/j.bone.2019.115141. Epub 2019 Nov 7. Bone. 2020. PMID: 31707108 Free PMC article.
-
Zoledronic acid increases the prevalence of medication-related osteonecrosis of the jaw in a dose dependent manner in rice rats (Oryzomys palustris) with localized periodontitis.Bone. 2018 Mar;108:79-88. doi: 10.1016/j.bone.2017.12.025. Epub 2017 Dec 28. Bone. 2018. PMID: 29289789 Free PMC article.
-
Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis.J Bone Miner Res. 2012 Oct;27(10):2130-43. doi: 10.1002/jbmr.1669. J Bone Miner Res. 2012. PMID: 22623376 Free PMC article.
-
Osteonecrosis of the Jaw in Patients Receiving Bone-Targeted Therapies: An Overview--Part I.Urol Nurs. 2016 May-Jun;36(3):111-6, 154. Urol Nurs. 2016. PMID: 27501591 Review.
-
Antiresorptives and osteonecrosis of the jaw.J Evid Based Dent Pract. 2012 Sep;12(3 Suppl):233-47. doi: 10.1016/S1532-3382(12)70046-5. J Evid Based Dent Pract. 2012. PMID: 23040351 Review.
Cited by
-
Anti-resorptive therapy in the osteometabolic patient affected by periodontitis. A joint position paper of the Italian Society of Orthopaedics and Traumatology (SIOT) and the Italian Society of Periodontology and Implantology (SIdP).J Orthop Traumatol. 2023 Jul 15;24(1):36. doi: 10.1186/s10195-023-00713-7. J Orthop Traumatol. 2023. PMID: 37453950 Free PMC article. Review.
-
Preclinical models of medication-related osteonecrosis of the jaw (MRONJ).Bone. 2021 Dec;153:116184. doi: 10.1016/j.bone.2021.116184. Epub 2021 Sep 11. Bone. 2021. PMID: 34520898 Free PMC article. Review.
-
Chronic Periodontal Infection and Not Iatrogenic Interference Is the Trigger of Medication-Related Osteonecrosis of the Jaw: Insights from a Large Animal Study (PerioBRONJ Pig Model).Medicina (Kaunas). 2023 May 22;59(5):1000. doi: 10.3390/medicina59051000. Medicina (Kaunas). 2023. PMID: 37241232 Free PMC article.
-
Effects of Puerarin Combined with PLGA/TCP/Puerarin on Osteocalcin and Sialoprotein of Mandibular Defects.Contrast Media Mol Imaging. 2022 Sep 6;2022:5177419. doi: 10.1155/2022/5177419. eCollection 2022. Contrast Media Mol Imaging. 2022. PMID: 36128172 Free PMC article.
-
Establishment and assessment of rodent models of medication-related osteonecrosis of the jaw (MRONJ).Int J Oral Sci. 2022 Aug 10;14(1):41. doi: 10.1038/s41368-022-00182-4. Int J Oral Sci. 2022. PMID: 35948539 Free PMC article. Review.
References
-
- Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, J. International Task Force on Osteonecrosis of the, Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus, J Bone Miner Res 30 (1) (2015) 3–23. - PubMed
-
- Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update, J. Oral Maxillofac. Surg 72 (10) (2014) 1938–1956. - PubMed
-
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A, Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, doubleblind study, J. Clin. Oncol 28 (35) (2010) 5132–5139. - PubMed
-
- Van den Wyngaert T, Wouters K, Huizing MT, Vermorken JB, RANK ligand inhibition in bone metastatic cancer and risk of osteonecrosis of the jaw (ONJ): non bis in idem? Support Care Cancer 19 (12) (2011) 2035–2040. - PubMed
-
- Nicolatou-Galitis O, Kouri M, Papadopoulou E, Vardas E, Galiti D, Epstein JB, Elad S, Campisi G, Tsoukalas N, Bektas-Kayhan K, Tan W, Body JJ, Migliorati C, Lalla RV, M.B.S. Group, Osteonecrosis of the jaw related to non-antiresorptive medications: a systematic review, Support Care Cancer 27 (2) (2019) 383–394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

