Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities
- PMID: 33510490
- PMCID: PMC7841384
- DOI: 10.1038/s41577-020-00488-6
Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities
Abstract
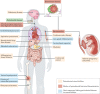
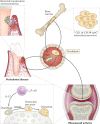
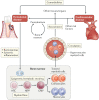
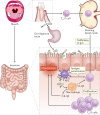
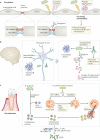
Periodontitis, a major inflammatory disease of the oral mucosa, is epidemiologically associated with other chronic inflammation-driven disorders, including cardio-metabolic, neurodegenerative and autoimmune diseases and cancer. Emerging evidence from interventional studies indicates that local treatment of periodontitis ameliorates surrogate markers of comorbid conditions. The potential causal link between periodontitis and its comorbidities is further strengthened by recent experimental animal studies establishing biologically plausible and clinically consistent mechanisms whereby periodontitis could initiate or aggravate a comorbid condition. This multi-faceted 'mechanistic causality' aspect of the link between periodontitis and comorbidities is the focus of this Review. Understanding how certain extra-oral pathologies are affected by disseminated periodontal pathogens and periodontitis-associated systemic inflammation, including adaptation of bone marrow haematopoietic progenitors, may provide new therapeutic options to reduce the risk of periodontitis-associated comorbidities.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications.Periodontol 2000. 2022 Jun;89(1):9-18. doi: 10.1111/prd.12430. Epub 2022 Mar 4. Periodontol 2000. 2022. PMID: 35244969 Free PMC article. Review.
-
Is periodontitis a comorbidity of COPD or can associations be explained by shared risk factors/behaviors?Int J Chron Obstruct Pulmon Dis. 2017 May 4;12:1339-1349. doi: 10.2147/COPD.S127802. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28496317 Free PMC article. Review.
-
Linking mechanisms of periodontitis to Alzheimer's disease.Curr Opin Neurol. 2020 Apr;33(2):230-238. doi: 10.1097/WCO.0000000000000797. Curr Opin Neurol. 2020. PMID: 32097126 Review.
-
The Molecular Comorbidity Network of Periodontal Disease.Int J Mol Sci. 2024 Sep 21;25(18):10161. doi: 10.3390/ijms251810161. Int J Mol Sci. 2024. PMID: 39337647 Free PMC article.
-
Infectious Triggers in Periodontitis and the Gut in Rheumatoid Arthritis (RA): A Complex Story About Association and Causality.Front Immunol. 2020 Jun 3;11:1108. doi: 10.3389/fimmu.2020.01108. eCollection 2020. Front Immunol. 2020. PMID: 32582191 Free PMC article. Review.
Cited by
-
Periodontitis and non-communicable diseases in a Brazilian population, a cross-sectional study, Vila Velha-ES, Brazil.Osong Public Health Res Perspect. 2024 Jun;15(3):212-220. doi: 10.24171/j.phrp.2024.0021. Epub 2024 Jun 27. Osong Public Health Res Perspect. 2024. PMID: 38988024 Free PMC article.
-
Topical Cellular/Tissue and Molecular Aspects Regarding Nonpharmacological Interventions in Alzheimer's Disease-A Systematic Review.Int J Mol Sci. 2023 Nov 20;24(22):16533. doi: 10.3390/ijms242216533. Int J Mol Sci. 2023. PMID: 38003723 Free PMC article. Review.
-
Prunin Laurate Derived from Natural Substances Shows Antibacterial Activity against the Periodontal Pathogen Porphyromonas gingivalis.Foods. 2024 Jun 18;13(12):1917. doi: 10.3390/foods13121917. Foods. 2024. PMID: 38928857 Free PMC article.
-
Association of Diet-Related Systemic Inflammation with Periodontitis and Tooth Loss: The Interaction Effect of Diabetes.Nutrients. 2022 Oct 3;14(19):4118. doi: 10.3390/nu14194118. Nutrients. 2022. PMID: 36235769 Free PMC article.
-
Alterations of HDL's to piHDL's Proteome in Patients with Chronic Inflammatory Diseases, and HDL-Targeted Therapies.Pharmaceuticals (Basel). 2022 Oct 18;15(10):1278. doi: 10.3390/ph15101278. Pharmaceuticals (Basel). 2022. PMID: 36297390 Free PMC article. Review.
References
-
- Genco RJ, Sanz M. Clinical and public health implications of periodontal and systemic diseases: an overview. Periodontol. 2000. 2020;83:7–13. - PubMed
-
- Potempa J, Mydel P, Koziel J. The case for periodontitis in the pathogenesis of rheumatoid arthritis. Nat. Rev. Rheumatol. 2017;13:606–620. - PubMed
-
- Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. 2000. 2020;83:90–106. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

