Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock
- PMID: 33505870
- PMCID: PMC7805252
- DOI: 10.5492/wjccm.v10.i1.22
Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb®) in patients with sepsis and septic shock
Abstract
Background: Sepsis is a severe clinical syndrome related to the host response to infection. The severity of infections is due to an activation cascade that will lead to an auto amplifying cytokine production: The cytokine storm. Hemoadsorption by CytoSorb® therapy is a new technology that helps to address the cytokine storm and to regain control over various inflammatory conditions.
Aim: To evaluate prospectively CytoSorb® therapy used as an adjunctive therapy along with standard of care in septic patients admitted to intensive care unit (ICU).
Methods: This was a prospective, real time, investigator initiated, observational multicenter study conducted in patients admitted to the ICU with sepsis and septic shock. The improvement of mean arterial pressure and reduction of vasopressor needs were evaluated as primary outcome. The change in laboratory parameters, sepsis scores [acute physiology and chronic health evaluation (APACHE II) and sequential organ failure assessment (SOFA)] and vital parameters were considered as secondary outcome. The outcomes were also evaluated in the survivor and non-survivor group. Descriptive statistics were used; a P value < 0.05 was considered to be statistically significant.
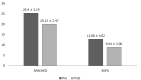
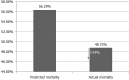
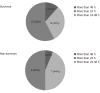
Results: Overall, 45 patients aged ≥ 18 and ≤ 80 years were included; the majority were men (n = 31; 69.0%), with mean age 47.16 ± 14.11 years. Post CytoSorb® therapy, 26 patients survived and 3 patients were lost to follow-up. In the survivor group, the percentage dose reduction in vasopressor was norepinephrine (51.4%), epinephrine (69.4%) and vasopressin (13.9%). A reduction in interleukin-6 levels (52.3%) was observed in the survivor group. Platelet count improved to 30.1% (P = 0.2938), and total lung capacity count significantly reduced by 33% (P < 0.0001). Serum creatinine and serum lactate were reduced by 33.3% (P = 0.0190) and 39.4% (P = 0.0120), respectively. The mean APACHE II score was 25.46 ± 2.91 and SOFA scores was 12.90 ± 4.02 before initiation of CytoSorb® therapy, and they were reduced significantly post therapy (APACHE II 20.1 ± 2.47; P < 0.0001 and SOFA 9.04 ± 3.00; P = 0.0003) in the survivor group. The predicted mortality in our patient population before CytoSorb® therapy was 56.5%, and it was reduced to 48.8% (actual mortality) after CytoSorb® therapy. We reported 75% survival rate in patients given treatment in < 24 h of ICU admission and 68% survival rates in patients given treatment within 24-48 h of ICU admission. In the survivor group, the average number of days spent in the ICU was 4.44 ± 1.66 d; while in the non-survivor group, the average number of days spent in ICU was 8.5 ± 15.9 d. CytoSorb® therapy was safe and well tolerated with no adverse events reported.
Conclusion: CytoSorb® might be an effective adjuvant therapy in stabilizing sepsis and septic shock patients. However, it is advisable to start the therapy at an early stage (preferably within 24 h after onset of septic shock).
Keywords: Acute physiology and chronic health evaluation score; Hemadsorption; Sepsis; Sequential organ failure assessment score; Vasopressor.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Experience with hemoadsorption (CytoSorb®) in the management of septic shock patients.World J Crit Care Med. 2020 Jan 31;9(1):1-12. doi: 10.5492/wjccm.v9.i1.1. eCollection 2020 Jan 31. World J Crit Care Med. 2020. PMID: 32104647 Free PMC article.
-
Hemoadsorption by extracorporeal cytokine adsorption therapy (CytoSorb®) in the management of septic shock: A retrospective observational study.Int J Artif Organs. 2020 Jun;43(6):372-378. doi: 10.1177/0391398819891739. Epub 2019 Dec 23. Int J Artif Organs. 2020. PMID: 31868078
-
Hemoadsorption by CytoSorb in septic patients: a case series.Crit Care. 2017 Mar 27;21(1):74. doi: 10.1186/s13054-017-1662-9. Crit Care. 2017. PMID: 28343448 Free PMC article.
-
International registry on the use of the CytoSorb® adsorber in ICU patients : Study protocol and preliminary results.Med Klin Intensivmed Notfmed. 2019 Nov;114(8):699-707. doi: 10.1007/s00063-017-0342-5. Epub 2017 Sep 4. Med Klin Intensivmed Notfmed. 2019. PMID: 28871441 Review. English.
-
Renal Replacement Techniques in Septic Shock.Int J Mol Sci. 2021 Sep 23;22(19):10238. doi: 10.3390/ijms221910238. Int J Mol Sci. 2021. PMID: 34638575 Free PMC article. Review.
Cited by
-
Hemoperfusion: technical aspects and state of the art.Crit Care. 2022 May 12;26(1):135. doi: 10.1186/s13054-022-04009-w. Crit Care. 2022. PMID: 35549999 Free PMC article. Review.
-
Role of Hemoperfusion With CytoSorb Associated With Continuous Kidney Replacement Therapy on Renal Outcome in Critically III Children With Septic Shock.Front Pediatr. 2021 Aug 24;9:718049. doi: 10.3389/fped.2021.718049. eCollection 2021. Front Pediatr. 2021. PMID: 34504817 Free PMC article.
-
Antimicrobial Exposure in Critically Ill Patients with Sepsis-Associated Multi-Organ Dysfunction Requiring Extracorporeal Organ Support: A Narrative Review.Microorganisms. 2023 Feb 13;11(2):473. doi: 10.3390/microorganisms11020473. Microorganisms. 2023. PMID: 36838438 Free PMC article. Review.
-
Cytokine hemoadsorption with CytoSorb® in patients with sepsis: a systematic review and meta-analysis.Crit Care Sci. 2023 Apr-Jun;35(2):217-225. doi: 10.5935/2965-2774.20230289-en. Crit Care Sci. 2023. PMID: 37712812 Free PMC article.
-
Use of the CytoSorb adsorber in patients with acute-on-chronic liver failure.Sci Rep. 2024 May 17;14(1):11309. doi: 10.1038/s41598-024-61658-3. Sci Rep. 2024. PMID: 38760460 Free PMC article.
References
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med. 2017;45:486–552. - PubMed
-
- van der Linde G, Grootendorst A. First case of toxic shock treated with haemoadsorption by CytoSorb® in the Netherlands. Neth J Crit Care. 2016;24:27–9.
-
- Divatia JV, Amin PR, Ramakrishnan N, Kapadia FN, Todi S, Sahu S, Govil D, Chawla R, Kulkarni AP, Samavedam S, Jani CK, Rungta N, Samaddar DP, Mehta S, Venkataraman R, Hegde A, Bande BD, Dhanuka S, Singh V, Tewari R, Zirpe K, Sathe P INDICAPS Study Investigators. Intensive Care in India: The Indian Intensive Care Case Mix and Practice Patterns Study. Indian J Crit Care Med. 2016;20:216–225. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

